Infrared spectroscopy for analysis of co-processed ibuprofen and magnesium trisilicate at milling and freeze drying
DOI:
https://doi.org/10.17344/acsi.2016.2772Keywords:
Infrared spectroscopy, co-milling, co-freeze drying, scanning electron microscopy, differential scanning calorimetry.Abstract
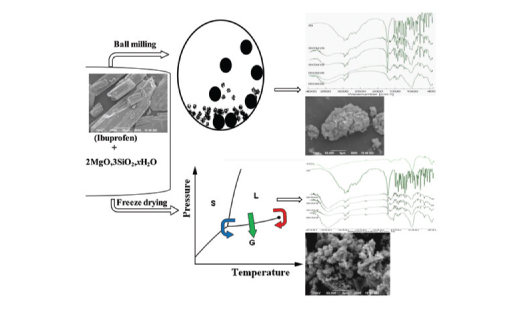
Assessment of interactions of ibuprofen and magnesium trisilicate after co-processing has been carried out by infrared spectroscopy. Dry-state ball-milling and, aqueous state kneading and freeze-drying were performed. FTIR spectroscopy of co-processed materials described acid–base reaction between the carboxylic acid containing ibuprofen to a significant extent. Increased absorbance of carboxylate peak accompanied by a consistently reduced absorbance of the carbonyl acid peak was evident. Absorbance of carboxylate peak was more in freeze-dried sample compared to milled product. Intermolecular hydrogen bonding between ibuprofen and magnesium trisilicate in the co-processed material has been suggested. Inhibition of crystal morphology has been noticed in the photomicrographs of both the products. DSC report has shown absence or significantly decreased melting endotherm representing almost complete amorphization of ibuprofen. Release of drug increased greatly after co-processing in comparison to crystalline ibuprofen. Freeze-dried samples have improved drug release more significantly compared to ball-milled samples.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
