Synthesis and Structure of [Cu(Hapn)]NO3]NO3, [Cu(Hapn)(H2O)2]SiF6, [Cu(Hapn)(H2O)BF4]BF4∙H2O and [Cu(Hapn)(NH2SO3)2] π-complexes (apn = 3-(prop-2-en-1-ylamino)propanenitrile)
DOI:
https://doi.org/10.17344/acsi.2016.3116Keywords:
Copper(I), π-complexes, aminonitrile derivative, crystal structure, coordination polymerAbstract
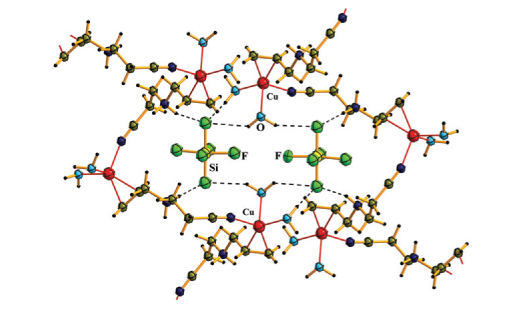
Four copper(I) π-complexes: [Cu(Hapn)NO3]NO3 (1), [Cu(Hapn)(H2O)2]SiF6 (2), [Cu(Hapn)(H2O)BF4]BF4·H2O (3) and [Cu(Hapn)(NH2SO3)2] (4) were prepared using alternating-current electrochemical technique, starting from alcohol solutions of 3-(prop-2-en-1-ylamino)propanenitrile (apn) titrated with appropriate acid and copper(II) salts (Cu(NO3)2∙3H2O, CuSiF6∙4H2O, Cu(BF4)2∙6H2O or Cu(NH2SO3)2∙xH2O, respectively). Obtained compounds were characterized by single-crystal X-ray diffraction and partially by IR spectroscopy. In the structures of complexes 1, 2 and 4 Cu(I) cation possesses a tetrahedral environment formed by the C=C bond of one organic cation Hapn, the N atom of ciano group from another Hapnmoiety, and two O atoms (from NO3– anions in 1, from H2O molecules in 2) or N atoms (NH2SO3– anions in 4). In compound 3 strongly pronounced trigonal-pyramidal coordination environment of Cu(I) is formed by a mid-point of C=C-bond of one Hapncation, nitrogen atom (of cyano group) of another Hapn unit, O atom of H2O molecule in the basal plane, and F atom of BF4– anion at the apical position.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
