Synthesis, cytotoxic and anti-proliferative activity of novel thiophene, thieno[2,3-b]pyridine and pyran derivatives derived from 4,5,6,7-tetrahydrobenzo[b]thiophene derivative
DOI:
https://doi.org/10.17344/acsi.2016.2920Keywords:
tetrahydrobenzo[b]thiophene, pyran, thiophene, cytotoxicity, anti-proliferative activityAbstract
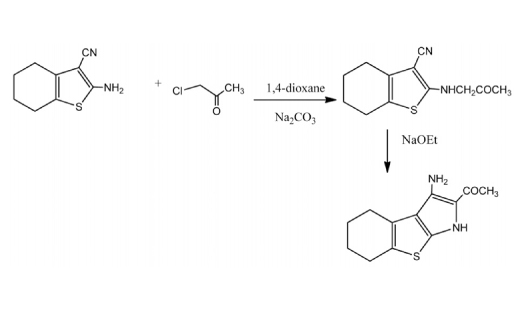
Novel tetrahydrobenzo[b]thienopyrole derivatives were synthesized from 2-amino-3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophene (1) through its reaction with α-chloroacetone to give the corresponding N-alkyl derivative 3. The latter underwent ready cyclization in sodium ethoxide solution to give the tetrahydrobenzo[b]thienopyrrole 4. The latter compound was used as the key starting material for the synthesis of thiophene, thieno[2,3-b]pyridine and pyran derivatives. The cytotoxicity of the synthesized products towards the human cancer cell lines namely gastric cancer (NUGC), colon cancer (DLD-1), liver cancer (HA22T and HEPG-2), breast cancer (MCF-7), nasopharyngeal carcinoma (HONE-1) and normal fibroblast (WI-38) cell lines was measured. Compounds 4, 7a, 7b, 8a, 8b, 10c, 10d, 10f, 12a, 12b, 14b and 15b exhibit the optimal cytotoxic effect against cancer cell lines. Compounds7b and 14b showed the maximum inhibitory effect and these are much higher than the reference CHS-828 (pyridyl cyanoguanidine). On the other hand, the anti-proliferative evaluations of these compounds with high potency against the cancer cell lines L1210, Molt4/C8, CEM, K562, K562/4 and HCT116 showed that compounds 7b and 8b gave IC50’s against Molt4/C8 and CEM cell lines higher than that of the reference, doxorubicin.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
