Synthesis and Structural Evaluation of 5-Methyl-6-acetyl Substituted Indole and Gramine
DOI:
https://doi.org/10.17344/acsi.2016.2911Keywords:
Indoles, Gramines, Crystal structure, Hydrogen bonds, C–H•••π interaction, π•••π interactionAbstract
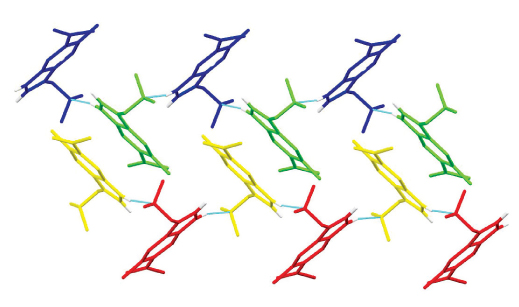
The synthesis and crystal structures of 1-(5-methyl-1H-indol-6-yl)ethan-1-one (7), C11H11NO, and 1-{3-[(dimethylamino)methyl]-5-methyl-1H-indol-6-yl}ethan-1-one (8), C14H18N2O, are reported. The synthesis is based on the Diels–Alder cycloaddition of a substituted 2H-pyran-2-one derivative, followed by an acid-catalyzed cyclization and concomitant deprotection (the last two steps were carried out as a one-pot domino process) yielding substituted indole 7, which was further derivatized via Mannich reaction to the gramine derivative 8. Both structures 7 and 8 were determined on the basis of IR, 1H NMR and mass spectroscopy, as well as by the elemental analysis and melting point determination. According to the single-crystal X-Ray diffraction analysis, the structure 7 has a single unique molecule in the asymmetric unit whereas the structure 8 contains four unique molecules in the asymmetric unit. Molecules 7 are linked via N–H···O hydrogen bonds between the secondary amine group and carbonyl moiety of the acetyl group of adjacent molecules, whereas molecules 8 are linked via N–H···N hydrogen bonds between the secondary and tertiary amine groups of adjacent molecules. Both structures are further stabilized by weak C–H···O, C–H···π and π···π interactions.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
