Application of “Click” chemistry in solid phase synthesis of alkyl halides
DOI:
https://doi.org/10.17344/acsi.2015.1478Keywords:
Iodination, polymer-bound triphenylphosphine, solid phase synthesis, benzylic alcohol, aliphatic alcohols, site-selectiveAbstract
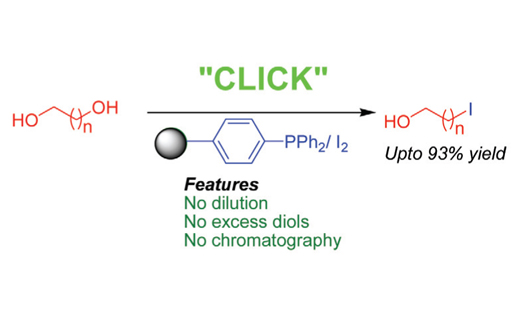
A convenient and highly selective microwave assisted procedure for the conversion of allylic, benzylic and aliphatic alcohols to their corresponding halide using polymer-bound triphenylphosphine and iodine is presented. In case of symmetrical diols, mono-iodination product is obtained in very high yield. Additionally, high regioselective behavior is observed in our procedure. Simplicity in operation, no column chromatography requirement for purification of the product, recyclability of the reagents used, short reaction times and good to excellent yields are the advantages of our protocol. Most functional groups remain unaffected under our reaction condition.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
