Synthesis of Novel Indole Derivatives, Antiproliferative Activity, Apoptosis, and Molecular Docking Studies
DOI:
https://doi.org/10.17344/acsi.2025.9406Abstract
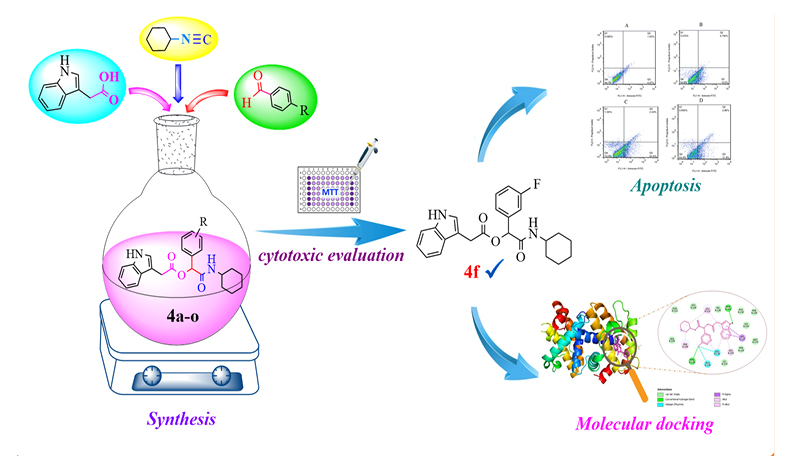
Novel indole-containing analogs were synthesized via a one-pot, multi-component Passerini reaction and subsequently evaluated for their anticancer activity against HeLa, MCF-7, and A549 cancer cell lines using the MTT assay. Among the synthesized compounds, (2-(cyclohexylamino)-1-(3-fluorophenyl)-2-oxoethyl 2-(1H-indol-3-yl)acetate (4f), which demonstrated the most potent cytotoxic activity, exhibited promising results with IC50 values of 17.71 and 19.92 μM against HeLa and MCF-7 cells, respectively. Flow cytometry analysis confirmed that compound 4f significantly induced apoptosis in HeLa cells in a concentration-dependent manner. Furthermore, molecular docking studies into the active site of the anti-apoptotic protein Bcl-xL indicated that compound 4f binds with good affinity, which is consistent with its considerable efficacy in the in vitro tests.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Dr., Zahra Sabzevari, Zakaria Forouzanfar, Ayyub Mojaddami

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
