Kinetic Parameters of Mineral Dissolution and Gibbs Free Energy
DOI:
https://doi.org/10.17344/acsi.2025.9348Abstract
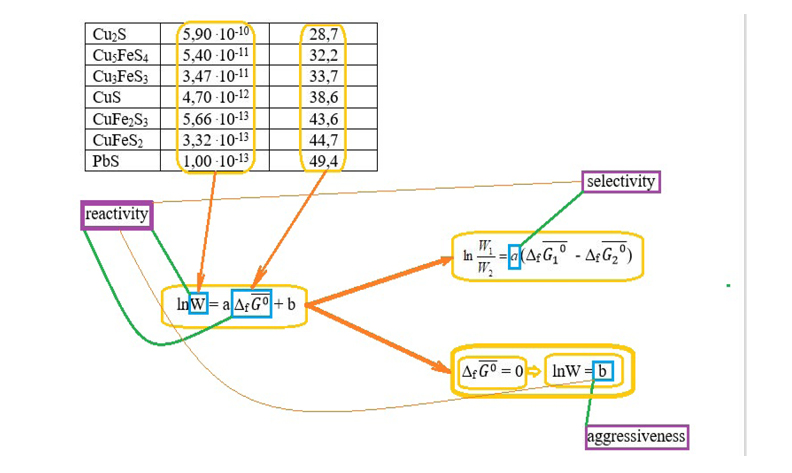
This study investigates the quantitative relationship between the kinetics and thermodynamics of mineral dissolution in heterogeneous reactions, specifically focusing on the dissolution of sparingly soluble minerals. Building on historical developments in chemical kinetics and thermodynamics, we propose empirical equations that describe experimental data and enable predictive modeling of dissolution processes. Two descriptors were evaluated: the normalized Gibbs energy of oxidation (ΔrG⁰/σ) and the average atomic Gibbs energy of mineral formation (ΔfG⁰/n). Experimental values of the apparent rate constant (k) and specific dissolution rate (W) were obtained under strictly controlled conditions. Linear regression models were developed. The regression coefficients were interpreted in terms of reagent selectivity and aggressiveness, consistent with the Bell–Evans–Polanyi principle. These findings support the use of ΔfG⁰/n as a reliable predictor of mineral reactivity and provide a thermodynamic basis for rational selection of leaching agents.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Alma Ryskaliyeva, Murat Baltabayev, Yerassyl Mukhamediyar, Aigul Zhubatova, Nurzhamal Khokhanbayeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
