Interpreting the Conformational Dynamics Over Interaction of FAM222A Protein with Amyloid-Beta Peptides
DOI:
https://doi.org/10.17344/acsi.2025.9340Abstract
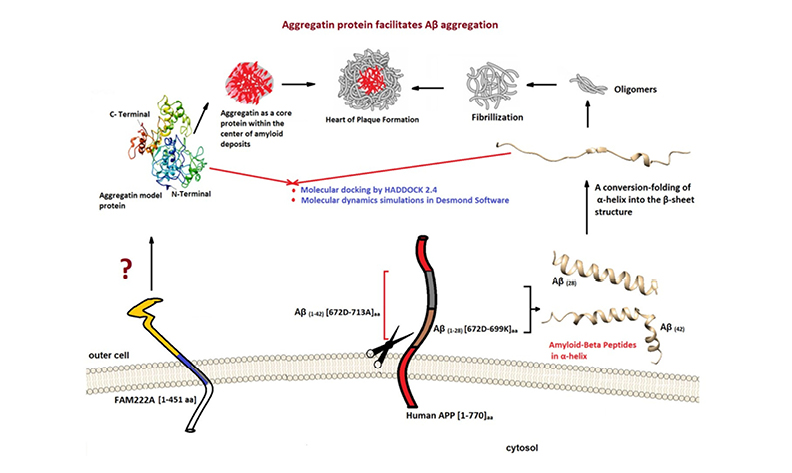
Alzheimer's disease (AD), a neurological disorder with increasing prevalence worldwide, presents a significant challenge to the medical community. The molecular mechanism underpinning its neuropathology is still wholly unexplained. Recent investigations have focused on the role of the protein aggregatin (AP), encoded by FAM222A, in amyloid-beta (Aβ) aggregation via its N-terminal Aβ binding domain. The current study aims to characterize the interaction mechanisms between the AP and Aβ (1-42 and 1-28) peptides using all-atom molecular dynamics (MD) simulations. The objective is to assess whether AP is a stabilizing scaffold in Aβ peptide aggregation, validate docking outcomes from previous studies, and compare the stability and interaction profiles of different Aβ isoforms. Aβ (1-42, 1-28) peptides were converted from the α-helix to the β-sheet form to inquire better-docked formation with the AP. The selected docking poses from our previous study and the four top scoring from HADDOCK were implemented in MD simulations, resulting in relatively stable complexes as indicated by consistent RMSD/RMSF trends without major structural disruptions. However, no binding free energy or interaction network analysis was conducted, and the conclusions are thus limited to structural stability observations. Our calculations hold accurate points for further experimental AD research on designing and developing the relevant protein-peptide interactions.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Nail Besli, Nilufer Ercin, Miguel Carmena-Bargueño, Horacio Pérez-Sánchez, Ulkan Celik

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
