Antityrosinase and Antimelanogenic Effects of Structurally Modified Kojic Acid Derivatives with Piperidine Side Chains
DOI:
https://doi.org/10.17344/acsi.2025.9318Abstract
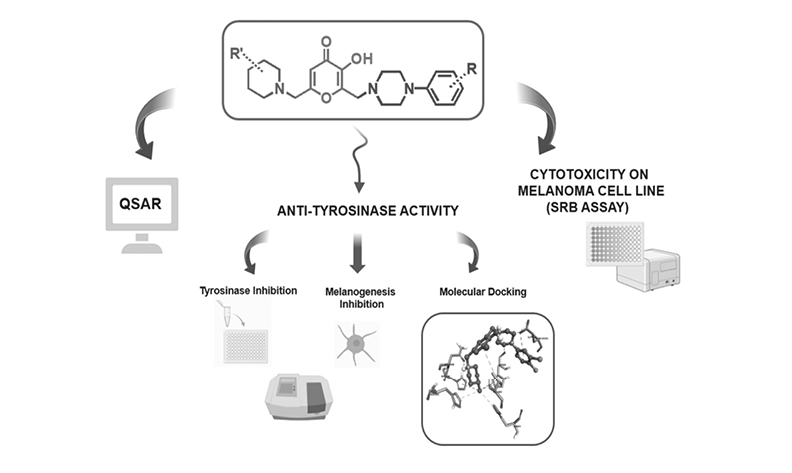
In this study, eight QSAR models were constructed to develop novel compounds as tyrosinase inhibitors. The decision tables, Bagging, and Random Committee methods showed the best predictive abilities (q2 ≥ 0.5) among the models. Based on these models, twelve new kojic acid derivatives were synthesized. Tyrosinase inhibition was determined using a spectrophotometric method with L-DOPA as the substrate. Molecular docking studies were conducted to provide insights into the tyrosinase-inhibiting mechanisms of the compounds. Cytotoxic effects on the B16F10 melanoma cell line were investigated using the SRB assay. A melanogenesis assay was also performed to detect the inhibition of melanin production. Compounds 4l, 4j, and 4b exhibited better tyrosinase inhibitory effects than the positive control, kojic acid (218.8 µM), with IC50 values of 138.1, 159.0, and 208.9 µM, respectively. Compound 4j showed the best anti-melanogenesis effect among the compounds tested. These findings demonstrate the potential of the compounds developed as novel tyrosinase inhibitors for clinical and cosmetic applications.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Gülşah Karakaya, Tugba Adak, Sami Hamdoun, Aslı Türe, Ayşe Ercan, Mutlu Aytemir

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
