Novel Mannich Bases Based on Schiff Bases: Synthesis, Biological Activities, Computational Approach, and Anticancer Analysis with Molecular Docking
DOI:
https://doi.org/10.17344/acsi.2025.9285Abstract
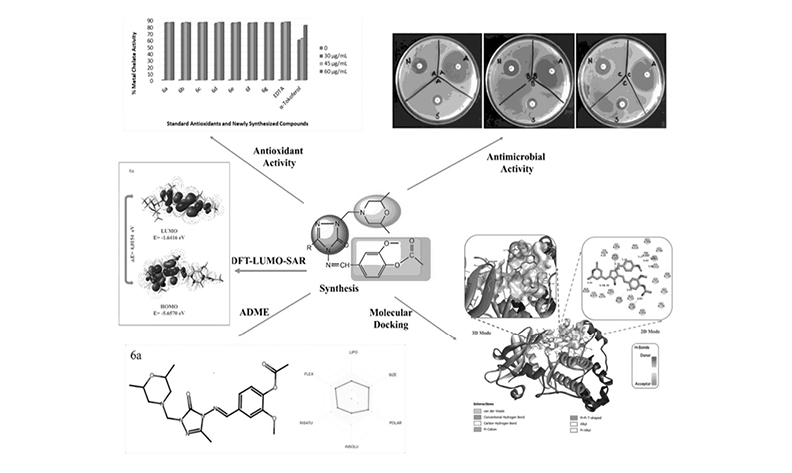
In this study, the Mannich base derivatives 1-(2,6-dimethylmorpholin-4-yl-methyl)-3-alkyl(aryl)-4-(3-methoxy-4-acetoxybenzylideneamino)-4,5-dihydro-1H-1,2,4-triazole-5-one 6(a-g) have been synthesized. The spectral analysis of the new compounds were identified utilizing 1H NMR, 13C NMR and IR spectrometry. Three techniques (Blois, Oyaizu, Dinis) were used to assess the potential antioxidant activities of the compound. Using the agar well diffusion method, the compounds' in vitro antibacterial properties were studied against six bacteria. Additionally, the molecular docking study was performed to research the potential anticancer activities of the compound against ovarian and gastric cancer. In molecular docking analysis, compound 6e gave good results in potential cancer interactions with protein 3W2S and compound 6f with protein 3OCB. Also, ADME estimations was performed to assess the drug-likeness of Mannich bases. The energies of molecular orbitals (HOMO-LUMO) and energy differ (ΔEg) was calculated for compounds. Finally, the structure-activity relationships (SAR) was analyzed by Density Functional Theory (DFT).
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Gül Kotan, Kenan Gören, Sevda Manap, Mehmet Bağlan, Haydar Yüksek

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
