Structural diversity of different aminomethylpyridinium hexafluoridosilicates
DOI:
https://doi.org/10.17344/acsi.2025.9194Abstract
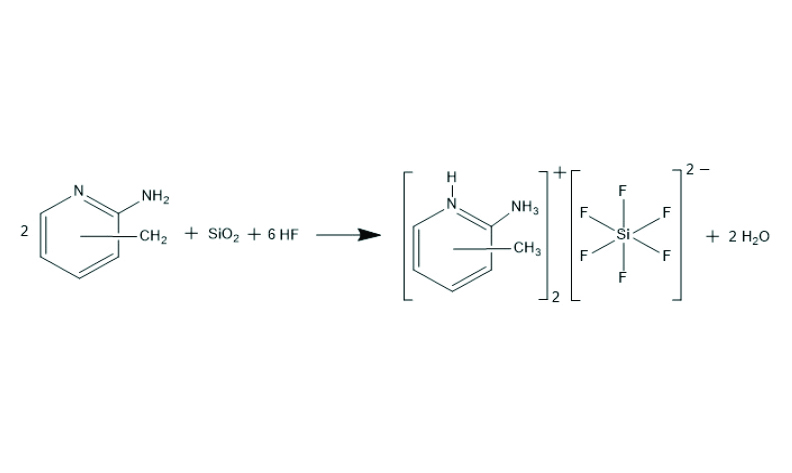
A series of new aminomethylpyridinium hexafluoridosilicate salts with the formula (RH)2[SiF6] (where R = 2-amino-3-methylpyridine (1), 2-amino-4-methylpyridine (2), 2-amino-5-methylpyridine (3) and 2-amino-6-methylpyridine (4)) were prepared by the reaction of various methyl-substituted 2-aminopyridines with hydrogen fluoride solution of silica. The crystal packing of these ionic salts is compared with respect to the position of the methyl group on the aromatic ring. The crystal structures are dominated by the non-covalent interactions: the N–H···F hydrogen bonds and π-π interactions between aromatic rings. The potential of the corresponding ionic salts to enable supramolecular associations was investigated. Compounds 1–4 were also characterized by 1H, 19F NMR and IR spectroscopy.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Andrej Pevec

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
