Crystal Structure Analysis and Anticancer Potential of a Naphthalene-Based Schiff Base Against Breast Cancer
DOI:
https://doi.org/10.17344/acsi.2025.9191Abstract
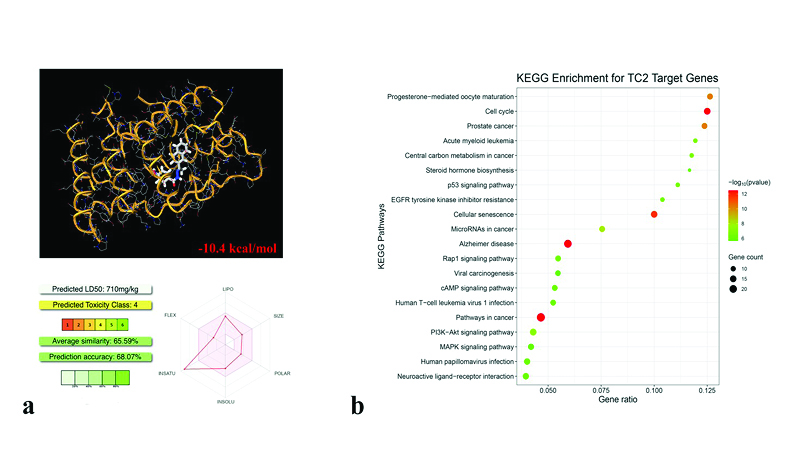
The crystal structure of the Schiff base compound 4-hydroxy-N-[(1Z)-1-(naphthalen-2-yl)ethylidene]benzohydrazide was determined using X-ray diffraction analysis, confirming the molecular structure previously inferred from spectroscopic data. The molecule exhibits nearly planar rings with specific dihedral angles. The crystal structure features intermolecular O–H···O and N–H···O hydrogen bonds that form a two-dimensional network, significantly stabilizing the structure. Hirshfeld surface analysis identified key intermolecular interactions, with notable contributions from H···C/C···H and H···H contacts. Energy calculations highlighted the dominant role of electrostatic interactions in the overall stability of the crystal. In vitro studies identified significant anticancer effects, with IC50 values of 38 µM for MCF7 cells and 57 µM for MDA-MB-231 cells, demonstrating dose-dependent inhibition of cell viability, migration, and clonogenic growth. In silico analyses revealed a strong binding affinity to ERRγ and predicted favorable oral bioavailability. KEGG pathway enrichment analysis of the predicted targets indicated their significant involvement in cancer-related pathways. The combined structural, in vitro, and in silico analyses provide a comprehensive understanding of the compound’s properties, laying a strong foundation for future preclinical and clinical studies.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Tolga Göktürk, Turan Demircan, Tuncer Hökelek, Mervenur Yavuz, Ramazan Güp, Cansu Topkaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
