Theoretical Study of the Stabilization of Lysine Through Pseudocyclic Conformation
DOI:
https://doi.org/10.17344/acsi.2024.9099Abstract
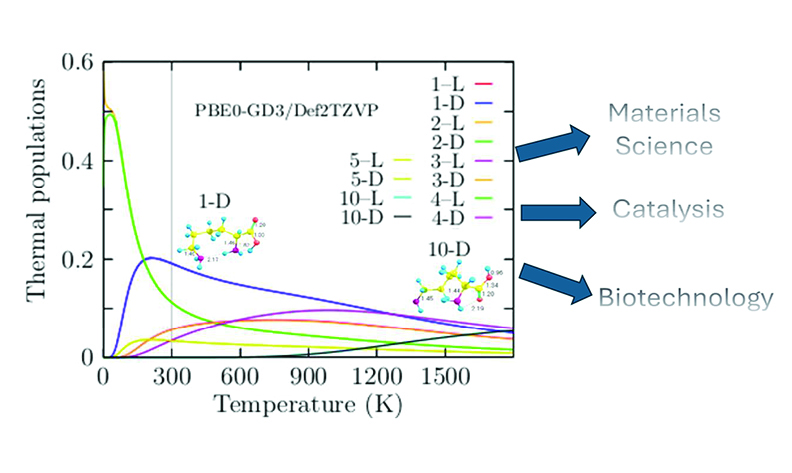
This study investigates lysine's conformational behavior and thermal properties at the PBE0-GD3/Def2-TZVP level for geometry optimizations and CCSD(T)/Def2-TZVP level of theory for the calculation of thermal population. Lysine forms pseudocyclic conformations stabilized by hydrogen bonds between amino and carboxyl groups. We identified 36 conformers, split evenly between L- and D-lysine, with small energy differences between structures. When the pseudocyclic conformation is disrupted, the energy is highly affected. Lysine’s geometries are remarkably sensitive to temperature. These findings highlight lysine’s temperature sensitivity and its potential for diverse applications. Its structural plasticity across 0-1800 K suggests roles in material science, such as temperature-responsive semiconductor thin films, coatings, sensors, catalysis through tunable active sites, and biomedicine for drug delivery or protein stabilization. The insights provided by this study emphasize lysine’s versatility as a biomolecule and its implications for technological innovation across multiple fields.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Germán Avila-Sierra, Aned de Leon, Ramón Ochoa-Landín, José Luis Cabellos, Santos Jesus Castillo

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
