Structures and properties of phenylethanoid glycosides from Barleria prionitis Linn.: Insights from theoretical and experimental investigations
DOI:
https://doi.org/10.17344/acsi.2024.9018Abstract
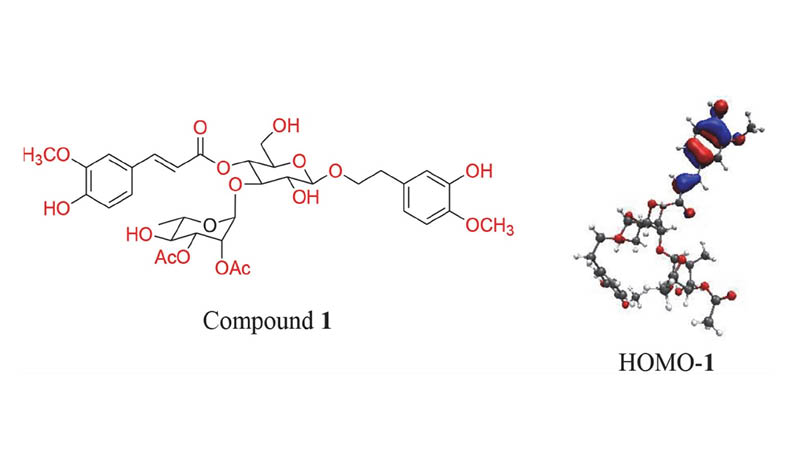
From the whole plant of Barleria prionitis Linn. collected in Laos, six phenylethanoid glycosides, acetylmartynoside A (1), martynoside (2), 3-O-methylpoliumoside (3), isoaceteoside (4), leucosceptoside A (5), 2-(3-hydroxy-4-methoxyphenyl)-ethyl-O-(α-l-rhamnosyl)-(1-3)-O-(α-l-rhamnos-yl)-(1-6)-4-O-E-feruloyl-β-d-glucopyranoside (6) were isolated. Their structures were elucidated by 1D and 2D NMR and mass spectra. In addition, the antioxidant activity of compounds 1-6 was investigated. All of the compounds showed Keap1 protein inhibitory effect with binding affinity in the range of -9.639 - -9.084 kcal/mol and moderate DPPH free radical scavenging activity with their IC50 values in the range of 110–389 μM. Furthermore, the predicted toxicity results of all of them indicated a level of 5 and a high LD50 value (LD50 = 5000 mg/kg), which showed low toxicity and high safety for oral consumption in humans.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Onesy Keomanykham, Hue Van Nguyen, Hoa Le Thi Phuong, Ha Nguyen Xuan, Bounnam Xangyaorn, Hue Minh Thi Nguyen, Quang Dang

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
