QSRR modeling of lipophilicity of new spirohydantoin derivatives determined with various TLC systems
DOI:
https://doi.org/10.17344/acsi.2023.8602Abstract
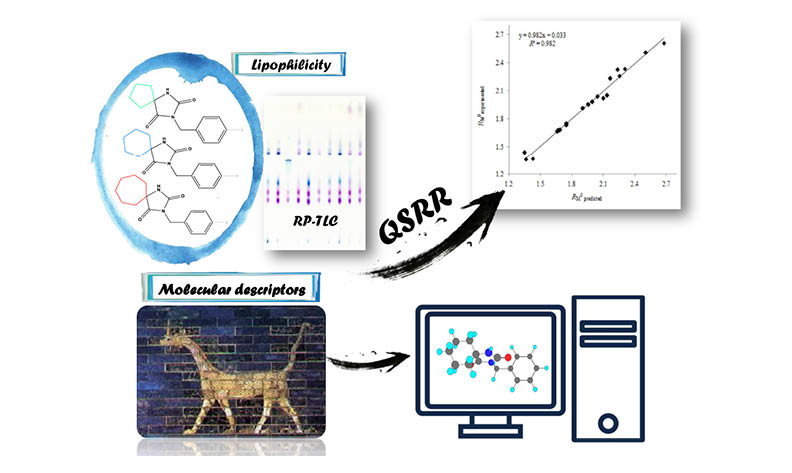
A Quantitative structure-retention relationship (QSRR) analysis has been carried out on the chromatography parameters of lipophilicity of selected spirohydantoins. Multiple linear regression (MLR) was applied for construct the QSRR models. The chromatographic parameters of lipophilicity were determined by reversed-phase thin-layer chromatography. Chromatographic analyses were performed on C-18 modified silica gel with a two-component mobile phase consisting of water and protic organic solvent (ethanol, n-propanol, i-propanol, or t-butanol) in different ratios. QSRR models were built and for additional four aqueous mobile phases: acetone-water, acetonitrile-water, tetrahydrofuran-water, and 1,4-dioxane-water (results published before). In total, chromatographic lipophilicity parameters obtained for two types of organic solvents was subject of the QSRR. The predictive ability of each model was defined by an internal validation coefficient. The best QSRR model for predicting the chromatographic parameter of lipophilicity was obtained for tetrahydrofuran as an organic solvent.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Kristina Tot, Anita Lazić, Tatjana Djaković Sekulić

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
