Synthesis of Novel cis-2-Azetidinones from imines and aclychloride using triethylamine
DOI:
https://doi.org/10.17344/acsi.2023.8451Abstract
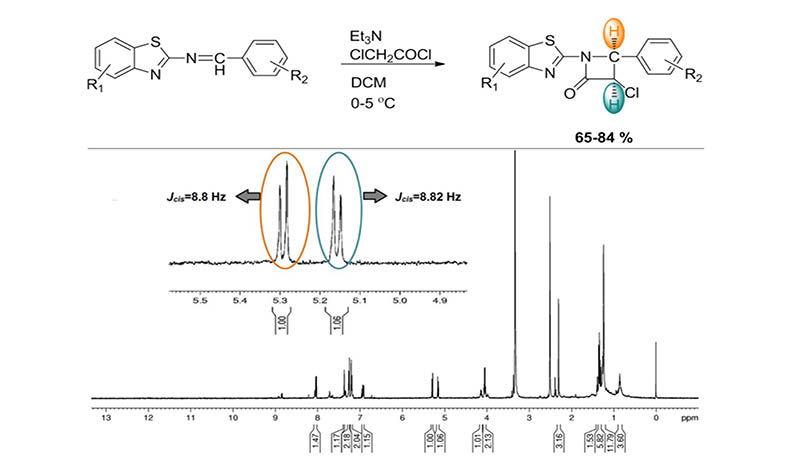
A novel series of cis-2-azetidinones 2(a-c ) was carried out by the cyclo addition reaction of imine 1(a–c ) and acyl chloride in dry dichloromethane at 0-5 oC using triphenylamine. The cyclo addition of the Schiff bases with chloroacetyl chloride resulted corresponding major product cis-2-azetidinone stereoisomers 2(a-c). The synthesized compounds were characterized by analytical and spectral (Infrared, 1H NMR, 13C NMR, and elemental analysis) data.
Downloads
Published
04.12.2023
Issue
Section
Organic chemistry
License
Copyright (c) 1970 Handan Can Sakarya, Aslı Ketrez

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
