Synthesis, structure characterization, DNA binding, and cleavage properties of mononuclear and tetranuclear cluster of copper(II) complexes
DOI:
https://doi.org/10.17344/acsi.2014.797Keywords:
copper complex, mononuclear, copper cluster, DNA binding, DNA cleavageAbstract
Abstract
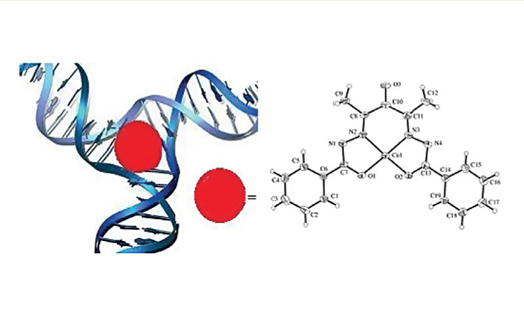
Two copper(II) complexes, cluster 1, and mononuclear 2, have been synthesized by reacting acetylacetone and benzohydrazide (1:1 ratio for 1 and 1:2 ratio for 2) with CuCl2 in a methanol solution. In 2, which is a new complex, the ligand acts as a tetradentate which binds the metal ion via two amide-O atoms and two imine-N atoms providing an N2O2 square-planar around the copper(II) ion. The absorption spectra data evidence strongly suggested that the two copper(II) compounds could interact with CT-DNA (intrinsic binding constant, Kb = 0.45×104 M-1 for 1 and Kb = 2.39×104 M-1 for 2). The super coiled plasmid pBR322 DNA cleavage ability was studied with 1 and 2 in the presence and absence of H2O2 as an oxidant. In both the absence and the presence of an oxidizing agent, complex 2 exhibited no nuclease activity. However, even in the absence of an oxidant, complex 1 exhibited significant DNA cleavage activity.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
