Synthesis, Characterization and DFT Studies of Two New π-conjugated Pyridine-based Tetrathiafulvalene Derivatives
Keywords:
Tetrathiafulvalenes, Pyridine, Synthesis, Crystal Structure, DFT calculationsAbstract
Two new π-conjugated pyridine-based tetrathiafulvalene derivatives, 2-(2- (4,5-bis(methylthio)-1,3-dithiol-2-ylidene)-6-phenyl-[1,3]dithiolo[4,5-b][1,4]dithiin-5-yl)pyridine (2a) and 3-(2-(4,5-bis(methylthio)-1,3-dithiol-2-ylidene)-6-(pyridin-2-yl) -[1,3]dithiolo[4,5-b][1,4]dithiin-5-yl)quinoline (2b), have been synthesized and characterized by 1H NMR, elemental analysis and mass spectroscopies. The compound 2a has also been studied by X-ray crystallography and theoretical calculations using density functional theory (DFT) framework with B3LYP/6-311+G(d,p) level of theory. Its crystal structure is triclinic system, space group P. The unit cell dimensions are: a = 8.813(3) Å, b = 11.082(3) Å, c = 12.620(4) Å, α = 88.805(5)°, β = 80.440(5)°, γ = 75.680(5)°, V = 1177.3(6) Å3, Z = 2. The molecule exhibits one classical C-H···N intermolecular hydrogen bonds, two kinds of short intermolecular S···S interactions and two types of C-H···π supramolecular interactions.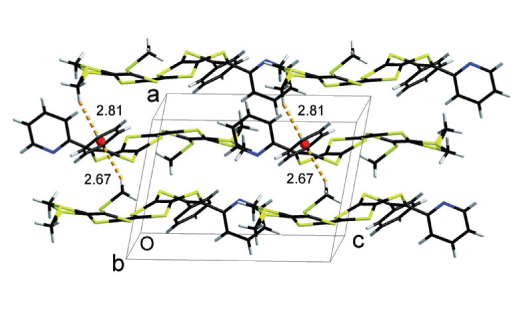
Downloads
Additional Files
Published
04.09.2014
Issue
Section
Organic chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
