Three 1D cyanide-bridged M(Ni, Pd, Pt)-Mn(II) coordination polymer: synthesis, crystal structure and magnetic properties
DOI:
https://doi.org/10.17344/acsi.2016.3132Keywords:
Cyanide-bridged, heterometallic complex, crystal structure, magnetic propertyAbstract
Three tetracyanide-containing building blocks K2[M(CN)4] (M = Ni, Pd, Pt) and one semi-closed macrocycle seven-coordinated manganese(II) compound have been employed to assemble cyanide-bridged heterometallic complexes, resulting in three cyanide-bridged MII-MnII complexes: [Mn(L)][Ni(CN)4]·2H2O (1) [Mn(L)][Pd(CN)4] (2) and [Mn(L)][Pt(CN)4] (3) (L = 2,6-bis[1-(2-(N-methylamino)ethylimino)ethyl]pyridine). Single-crystal X-ray diffraction analysis shows their similar one-dimensional structure consisting of the alternating [Mn(L)]2+ species and [M(CN)4]2– building blocks, generating a cyanide-bridged neutral polymeric chain. In all three isostructural complexes the coordination geometry of manganese ion is a slightly distorted pentagonal-bipyramidal with the two cyanide nitrogen atoms at the trans positions and N5 coordinating mode at the equatorial plane from ligand L. Investigation over magnetic properties of these complexes reveals very weak antiferromagnetic interaction between neighboring Mn(II) ions bridged by the long NC–M–CN unit. A best-fit to the magnetic susceptibility of complexes 1–3 leads to the magnetic coupling constant of J = –0.081, –0.103 and –0.14 cm–1, respectively.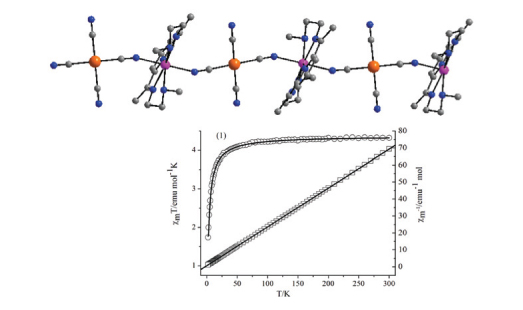
Additional Files
Published
21.02.2017
Issue
Section
Inorganic chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
