Effect of ZnO on the thermal degradation behavior of poly(methyl methacrylate) nanocomposites
DOI:
https://doi.org/10.17344/acsi.2016.2324Keywords:
ZnO-PMMA nanocomposites, thermal properties, activation energy, electron microscopy, thermogravimetric analysis (TGA)Abstract
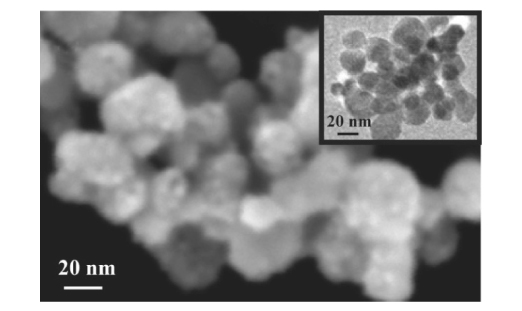
The influence of ZnO nanoparticles on the thermal degradation behavior of poly(methyl methacrylate) (PMMA) was tested using thermoanalytical techniques. The studied materials were investigated using TG, DTA, EGA, XRD, SEM and TEM. The ZnO nanoparticles were synthesized via precipitation by adding LiOH into Zn2+ water/ethylene glycol solutions. The ZnO-PMMA nanocomposites were prepared by adding the appropriate amount of ZnO into MMA and subsequent MMA radical polymerization. According to the experimental results and model-free isoconversional activation energy calculations, the addition of ZnO into PMMA played a double role. The ZnO concentrations up to 0.15% stabilized the composite by shifting the degradation interval toward higher temperatures and increasing the apparent activation energy relative to pure PMMA. At higher concentrations, the catalytic effect of ZnO started to prevail and was reflected in the lower temperature intervals of intense PMMA degradation and lower apparent activation energy. The addition of ZnO generally did not change the nature of the PMMA decomposition process.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
