Heteroannelation of cyclic ketones: Synthesis, characterization and antitumor evaluation of some condensed azine derivatives
DOI:
https://doi.org/10.17344/acsi.2016.2297Keywords:
Cyclopentapyrimidine, Thiazolopyrimidine, Quinazoline, Chromene, Antitumor activityAbstract
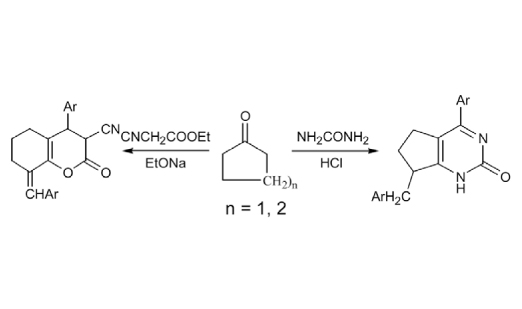
A series of pyrimidine and thiazine derivatives synthesized by one pot reaction of cyclopentanone with a mixture of an aromatic aldehyde namely o-anisaldehyde and different ureas namely urea, guanidine and thiourea, respectively. Furthermore, cycloaddition reaction of active methylene reagents namely acetyl acetone, malononitrile, ethyl cyanoacetate, cyanoacetamide and N-phenyl cyanoacetamide with 2,6-bis(2-methoxybenzylidene)-cyclohexanone afforded chromene and quinoline derivatives in basic medium. The antitumor evaluation of some new compounds against three human cell lines namely MCF-7, NCI-H460 and SF-268 showed significant and moderate activity compared with the positive control doxorubicin.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
