Basic hydrolysis of fullerene-based esters: a tiny step away from nucleophilic addition to fullerene
DOI:
https://doi.org/10.17344/acsi.2015.1440Keywords:
Th-symmetric Fullerenehexamalonic acid, fullerenol, basic hydrolysis, UV/Vis spectrophotometry, IR spectroscopyAbstract
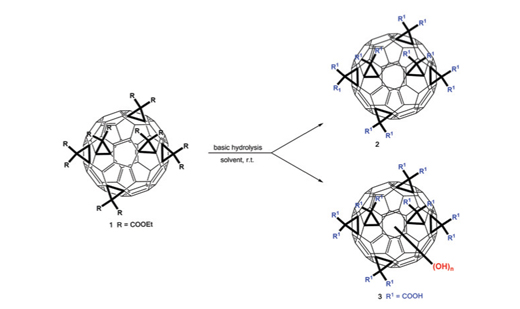
Direct nucleophilic addition of –OH groups on the C60 skeleton in the basic hydrolysis of ethyl ester of Th-symmetric fullerenehexamalonic acid (Th-FHMA), leading to the formation of a hybrid with features of Th- FHMA and fullerenol, have been observed as an important side reaction. The hydroxylation takes place at considerably milder conditions as those usually used in the synthesis of C60 fullerenols. UV/Vis and IR spectroscopy were successfully used as a fast monitoring tool which might be of help also in other investigations where additions on C60 skeleton of molecules with distinct absorption spectra takes place.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
