Synthesis, X-Ray Crystal Structures and Urease Inhibitory Activity of 3,5-Dihydroxy-N’-(pyridin-2-ylmethylene)benzohydrazide and its Copper(II) and Nickel(II) Complexes
DOI:
https://doi.org/10.17344/acsi.2025.9518Abstract
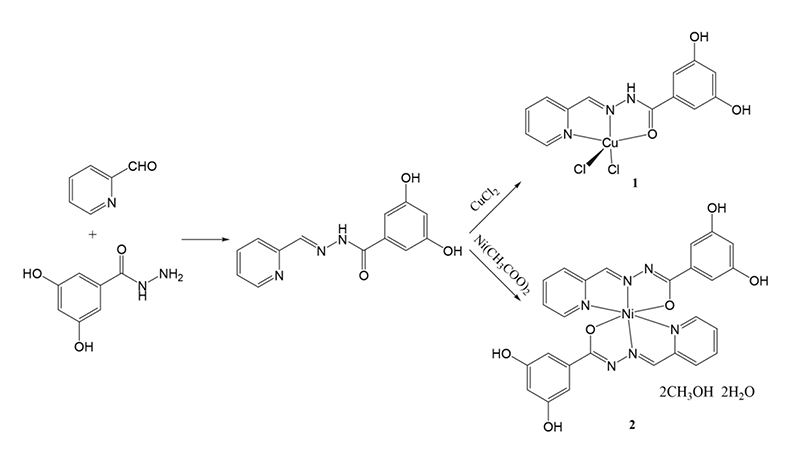
Hydrazone compounds have interesting biological activities. In this work, a new hydrazone compound 3,5-dihydroxy-N’-(pyridin-2-ylmethylene)benzohydrazide (HL) was synthesized and characterized by IR, UV-Vis, 1H and 13C NMR spectroscopy. The compound reacts with copper chloride and nickel acetate, respectively, to afford metal complexes [CuCl2(HL)]∙CH3OH (1∙CH3OH) and [NiL2]∙2CH3OH∙2H2O (2∙2CH3OH∙2H2O). The complexes were characterized by elemental analysis, and IR and UV-Vis spectroscopy. Structures of HL and the complexes were further confirmed by single crystal X-ray determination. The hydrazone ligand in complex 1 adopts neutral form and coordinates to Cu ion through pyridine nitrogen, imino nitrogen and carbonyl oxygen atoms. The hydrazone ligands in complex 2 adopt monoanionic form and coordinate to Ni ion through pyridine nitrogen, imino nitrogen and enolate oxygen atoms. The Cu atom in complex 1 is in square pyramidal coordination, and the Ni atom in complex 2 is in octahedral coordination. The compounds were tested for their urease inhibitory activities. Complex 1 has remarkable activity on Jack bean urease (IC50 = 0.5 ± 0.1 μmol L–1).
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Zhonglu You, Ziyi Qiao, Yaoyao Cao, Dahua Shi, Niansui Song, Xinhui Feng

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
