Synthesis, X-ray Crystal Structures and Antibacterial Activity of Copper(II) Complexes Derived from Halo Substituted Hydrazones
DOI:
https://doi.org/10.17344/acsi.2025.9497Abstract
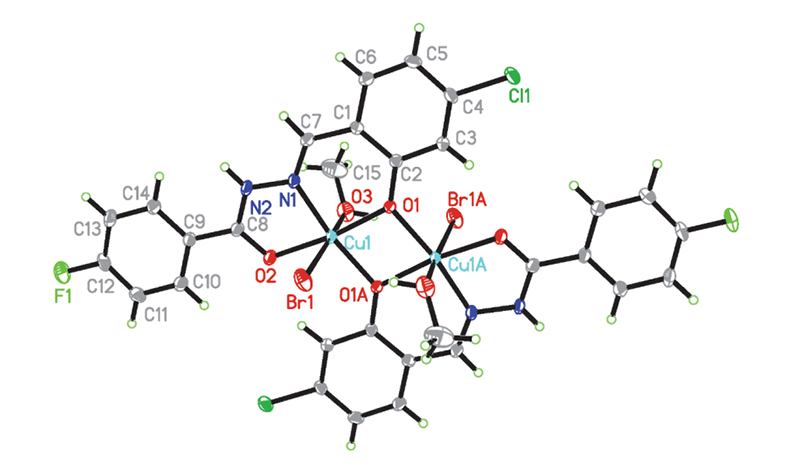
Schiff bases bearing halo substituent groups have interesting biological activities. In this work, two new hydrazone type Schiff bases N’-(3,5-dibromo-2-hydroxybenzylidene)-3-methylbenzohydrazide (HL1) and N’-(4-chloro-2-hydroxybenzylidene)-4-fluorobenzohydrazide (HL2), bearing bromo, chloro and fluoro substituent groups were synthesized. The compounds reacts with various copper salts to form three copper(II) complexes with interesting structures. The complexes are [CuBrL1]∙H2O (1∙H2O), [CuClL2]·CH3OH (2·CH3OH) and [Cu2Br2L22(CH3OH)2] (3). The hydrazones and three copper complexes were characterized by physico-chemical methods such as elemental analysis, infrared and electronic spectroscopy. The free hydrazones were also characterized by 1H NMR spectroscopy, and structures of H2L1 and the complexes were further confirmed by single crystal X-ray determination. The hydrazone ligands in the complexes coordinate to Cu ions through phenolate oxygen, imino nitrogen and carbonyl oxygen atoms. The Cu ion in complex 1 is in square planar coordination, in complex 2 is in square pyramidal coordination, and in complex 3 is in octahedral coordination. The complexes show good antibacterial activities on the bacterial strains Bacillus subtilis, Staphylococcus aureus and Escherichia coli, while weak activity on the fungal strain Candida albicans and no activity on Aspergillus niger.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Zhonglu You, Niansui Song, Ziyi Qiao, Shiyu Zhang, Wanlin Wei, Helin Wang, Yuqing Gu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
