Cyclopentyl-linked N-Acylthioureas as Promising Urease Inhibitors: Insights from in vitro Bioassay, Structure-activity Relationships and Computational Analysis
DOI:
https://doi.org/10.17344/acsi.2025.9469Abstract
Abstract
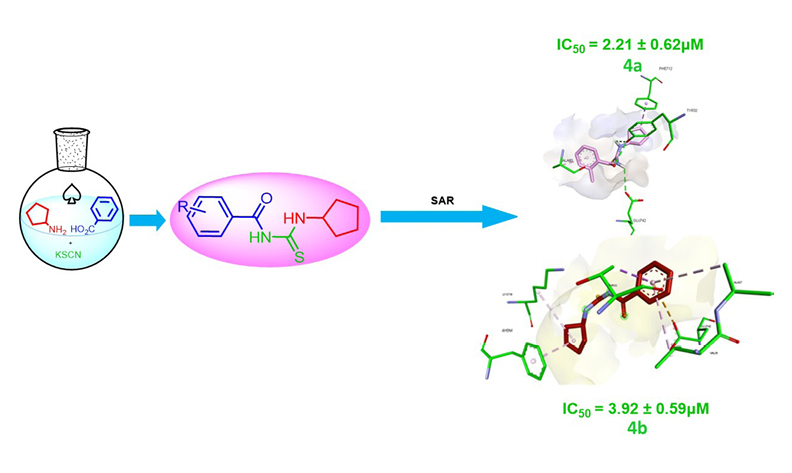
Helicobacter pylori is a Gram-negative bacteria responsible for gastrointestinal disorders, including chronic gastritis and potentially life-threatening conditions like gastric cancer. To manage these adverse outcomes, inhibiting the urease enzyme emerges as a promising strategy. A concise set of cyclopentyl-bearing N-acylthioureas 4a–j was synthesized, characterized and assessed for their ability to inhibit urease enzyme. All the tested compounds exhibited urease inhibitory activities, displaying superior enzyme inhibition when compared to the standard, thiourea (IC50 values 23.00 ± 0.03 μM). 4a and 4b exhibited the highest inhibitory efficacy with IC50 values of 2.21 ± 0.62 and 3.92 ± 0.59 μM, respectively. Both compounds demonstrated ≈10- and ≈6-folds superior inhibition than standard inhibitor, respectively. Moreover, molecular docking investigations revealed crucial interactions between potent ligands and active site residues. Molecular dynamics simulations and ADME properties revealed ligand-protein stability and druglikeness behavior of potent leads paving the way for treatment for gastritis.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Khansa Mumtaz, Sumera Zaib, Aamer Saeed, Atteeque Ahmed, Afifa Tur Rehman, Aneeza Asghar, Imtiaz Khan

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
