Synthesis, Characterization, and Theoretical Calculation of a Copper Complex of 3-Hydroxy-2-methylquinolin-4- carboxylate and 1,10-Phenanthroline
DOI:
https://doi.org/10.17344/acsi.2025.9384Abstract
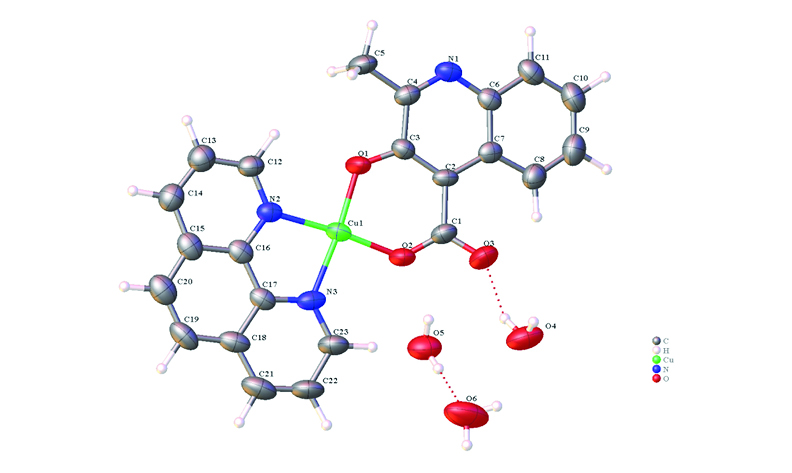
A copper complex containing mixed ligands [Cu(MCA)(Phen)·3H2O)] (HMCA = 3-hydroxy-2-methylquinoline-4-carboxylic acid, Phen = 1,10-phenanthroline) was prepared by the hydrothermal method. Structure was characterized by single crystal X-ray diffraction. Solid state fluorescence photoluminescence measurement shows a strong emission peak at 620 nm, which is attributed to the characteristic electronic transitions and molecular stacking effects within the ligand. CIE color difference analysis indicates that the title complex exhibits red photoluminescence (chromaticity coordinates of 0.1256, 0.2418). In addition, solid-state UV-Vis diffuse reflectance experiments revealed that the titled complex has an energy band gap of 1.578 eV.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Yan-Hua Li, Xing-Jian Liu, Jian Huang, Yu Xie, Fei Deng, Xiu-Guang Yi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
