Fibrous Silica KCC-1 as a Platform for Mn-Based Dual Metal Oxide Adsorbents for CO2 Capture
DOI:
https://doi.org/10.17344/acsi.2025.9370Abstract
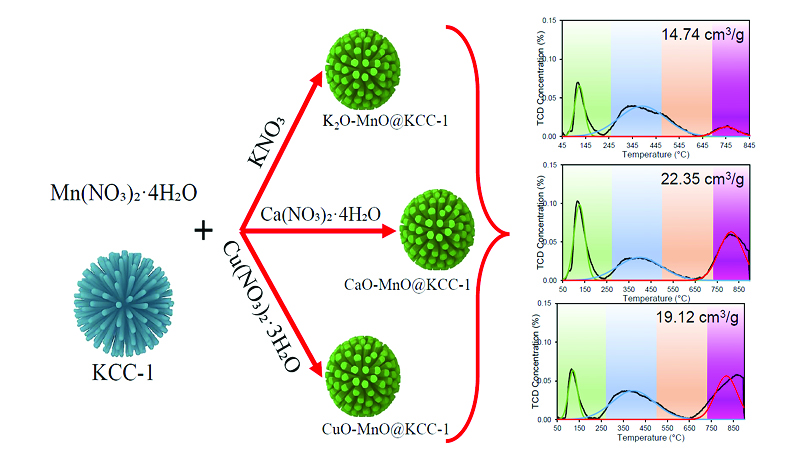
The continuous rise in atmospheric CO2 levels due to industrial emissions and fossil fuel combustion has intensified the need for efficient carbon capture. Solid adsorbents are favoured for their reusability and low energy demand, yet often face limitations in thermal stability and adsorption performance. This study examines the effect of co-loading manganese (Mn) with potassium (K), copper (Cu), and calcium (Ca) on fibrous silica KCC-1 for CO2 capture over a wide temperature range. KCC-1 was synthesised via a microemulsion method, and metals were introduced using an ultrasonic-surfactant-assisted impregnation technique. Characterisation using XRD, FTIR, BET, FESEM-EDX, and CO2-TPD confirmed structural integrity, surface functionality, and adsorption behaviour. CaO-MnO@KCC-1 shows the most balanced textural properties and the highest CO2 uptake due to its strong basicity and varied adsorption site strength. This highlights its potential as a temperature-flexible CO2 adsorbent.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Syawal Mohd Yusof, Azizul Hakim Lahuri, Nurul Asikin Mijan, Umar Kalmar Nizar, Siti Sarahah Sulhadi, Salma Samidin, Ainil Hafiza Abdul Aziz

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
