Copper(I/II) and Palladium(II) Complexes Containing Carbothioamide and Triphenylphosphine Ligands: Synthesis, Characterization, and Theoretical Studies
DOI:
https://doi.org/10.17344/acsi.2025.9352Abstract
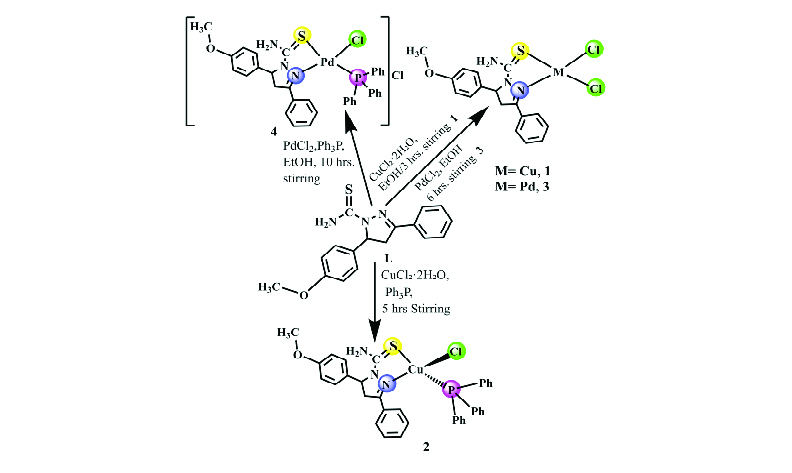
A carbothioamide ligand, 4,5-dihydro-5-(4-methoxyphenyl)-3-phenylpyrazole-1-carbothioamide, [C17H17N3OS], has been synthesized from the condensation of 4-methoxychalcone with thiosemicarbazide. The carbothioamide (L) ligand and triphenylphosphine (Ph3P) as co-ligand, was coordinated with Cu(I), Cu(II), and Pd(II) metal ions to synthesis the corresponding complexes: [CuCl2(L)] 1, [CuCl(L)(Ph3P)] 2, [PdCl2(L)] 3, and [PdCl(L)(Ph3P)]Cl 4. The ligand and all complexes were collected in solid form after the reactions and characterized by magnetic susceptibility, elemental analysis, molar conductivity, FT-IR, UV-Vis, and 1H, 13C, 31P-NMR techniques. The molar conductance values in DMSO (5.8–16.3 Ω–1 cm2 mol–1) confirmed all the complexes to be non-electrolytic except for the Pd(II) complex 4 (32.6 Ω–1cm2 mol–1) that behaves as a 1:1 electrolyte. According to spectroscopic evidence, the carbothioamide ligand behaves as an N, S donor and chelating agent. Magnetic susceptibility measurements combined with electronic spectral data suggest that the Cu(II) and Pd(II) complexes have square planar geometry, whereas the Cu(I) complex 2 has a tetrahedral geometry. Elemental analysis and 1H-NMR spectroscopy confirmed the mononuclear structure of all complexes. DFT calculations showed that the synthesized complexes 1, 3, and 4 exhibit higher thermodynamic stability than the free ligand (L), with ΔE values of 1.4695, 2.1116 eV, 1.9076 eV, and 1.2980 eV, respectively. In contrast, complex 2 has ΔE = 0.5385 eV, indicating lower thermodynamic stability. Among the complexes, complex 2 (S = 3.7140 eV) exhibited the highest softness, and all complexes were observed to be softer than the triphenylphosphine ligand. According to the results, electron transitions are easier in certain complexes than in their ligands, which suggests that the prepared complexes could be used in the photocell in future studies.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Karwan Omer Ali, Nabil Adil Fakhre , Salim Najm Aldain Saber

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
