Structure of 7,9,12,15,18,20,39,24,45,57-C60(CF3)10(1,2:3,4-O)2. The First Regiospecific Diepoxidation of a Fullerene Derivative
Keywords:
Fullerene, epoxide, X-ray structure, fulvene, diepoxidation, 19F NMRAbstract
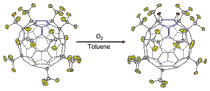
An unusual regiospecific diepoxidation of an isomer of C60(CF3)10 was discovered to slowly occur in solution while exposed to air. This is the only known example of a perfluoroalkyl fullerene undergoing epoxidation under ambient conditions. This compound was characterized by a variety of analytical methods including 19F NMR spectroscopy, APCI-MS, UV-vis spectroscopy, and X-ray crystallography. Numerous oxidation methods were explored including ozonation, photolysis, thermolysis, and the chemical oxidation by m-chloroperoxobenzoic acid, none of which has yielded the diepoxide. This indicated that the process is kinetically very slow, presumably due to steric crowding around the oxygen addition sites. These findings of spontaneous diepoxidation of the otherwise stable compound have far reaching implications as more fullerenes and fullerene derivatives are being used in organic electronics, and their instability toward oxidation in ambient conditions may alter the performance of the device.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
