N,N’-Bis(4-Bromo-2-hydroxybenzylidene)ethylenediamine and Its Copper(II), Zinc(II) and Cobalt(III) Complexes: Synthesis, Crystal Structures and Urease Inhibition
DOI:
https://doi.org/10.17344/acsi.2025.9224Abstract
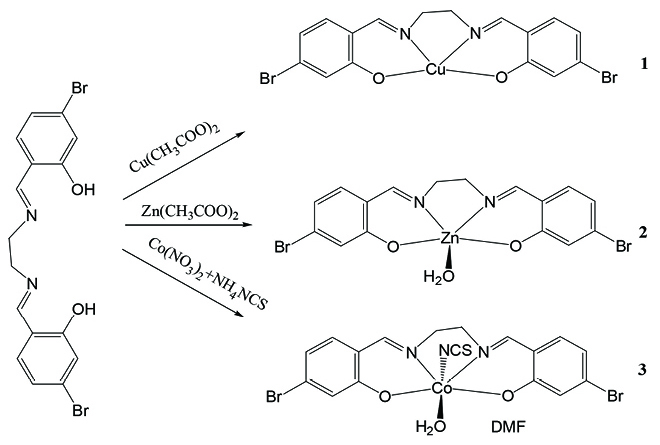
A new Schiff base compound N,N’-bis(4-bromo-2-hydroxybenzylidene)ethylenediamine (H2L) was prepared by reaction of 4-bromosalicylaldehyde with ethane-1,2-diamine in methanol. Reaction of H2L with copper acetate and zinc acetate in methanol, respectively, afforded two mononuclear complexes [CuL] (1) and [ZnL(OH2)] (2). Reaction of H2L with cobalt nitrate in the presence of ammonium thiocyanate in a mixture of methanol and DMF afforded a mononuclear cobalt(III) complex [CoL(NCS)(OH2)]·DMF (3). The free Schiff base and the complexes were characterized by elemental analysis and infrared spectra, as well as single crystal X-ray determination. 1H NMR spectrum was also performed for H2L. The deprotonated form of the Schiff base coordinates to the metal atoms through all the phenolate oxygen and imino nitrogen atoms. The Cu(II) atom in complex 1 is in square planar coordination. The Zn(II) atom in complex 2 is in square pyramidal coordination. The Co(III) atom in complex 3 is in octahedral coordination. The urease inhibitory assay reveals that the copper(II) complex has the most effective activity.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Rundong Lu, Jinkai Lei, Jiacheng Liu, Chi Liu, Liuxiu Chen, Wu Chen

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
