Synthesis and Characterization of Tetraarylphenazine and Tetraaryldibenzo[b,i]phenazine
DOI:
https://doi.org/10.17344/acsi.2025.9186Abstract
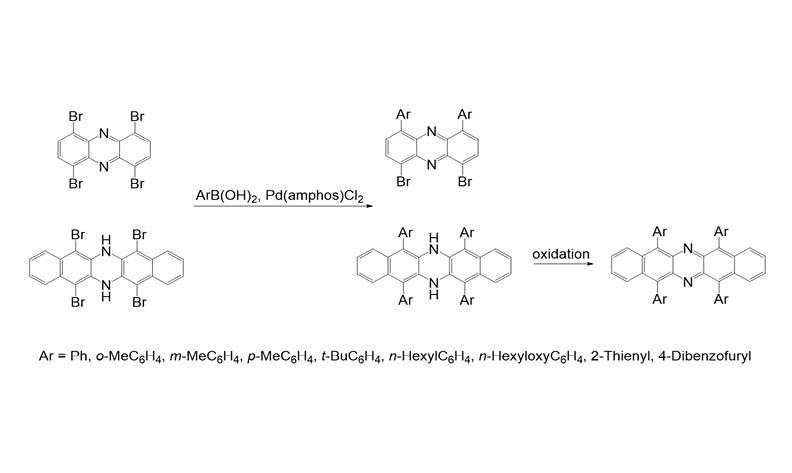
Phenazine and 6,13-diazapentacene are nitrogen-containing linear heterocycles that have attracted significant attention in the fields of organic electronics, and materials science. A straightforward strategy has been developed to construct 1,4,6,9-tetraarylphenazines and 5,7,12,14-tetraaryl-6,13-diazapentacenes with 1,4,6,9-tetrabromophenazine and 5,6,12,14-tetrabromo-6,13-dihydrodibenzo[b,i]phenazine as the key intermdiates, which were obtained easily from the bromination of phenazine and 6,13-dihydrodibenzo[b,i]phenazine, respectively. While the Pd-132 catalyzed ligand-free Suzuki couplings between above ascribed intermediates and various arylboronic acids were performed to afford directly tetraarylphenazines or tetraaryl-6,13-diazapentacenes by sequent oxidations. All of the new compounds are fully characterized by spectroscopy.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Liang-Hong Hu, Qian Cai Liu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
