Appliance Features of 4-Amino-1,2,4-triazole-3-thiols in the Synthesis of 3,6-Disubstituted [1,2,4]Triazolo[3,4-b][1,3,4]thiadiazoles: A Review
DOI:
https://doi.org/10.17344/acsi.2025.9169Abstract
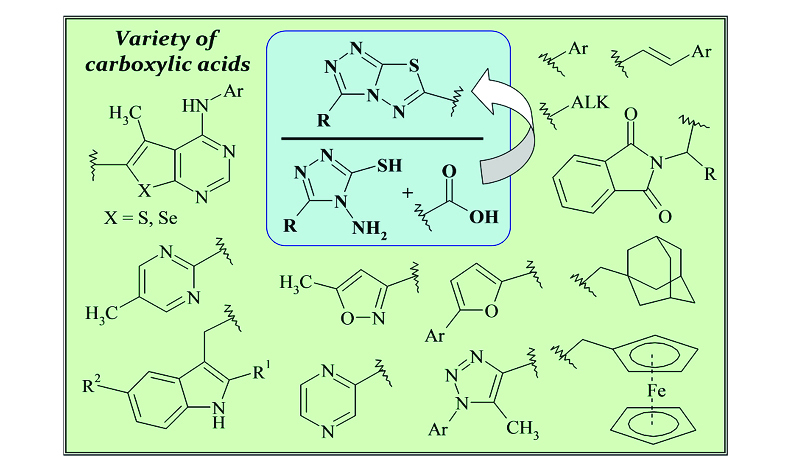
4-Amino-5-substituted-4H-1,2,4-triazole-3-thiols are versatile synthons for constructing of various biologically active heterocycles. This is provided by the close proximity of the amino and mercapto groups, which serve as readily accessible nucleophilic centers for the preparation of N-bridged heterosystems. One of the possible and convenient directions of using 4-amino-4H-1,2,4-triazole-3-thiols in heterocyclic synthesis based on their utilization in reaction with various carboxylic acids in the presence of dehydrating reagents, most often phosphorus oxochloride. Synthesized as follows [1,2,4]triazolo[3,4-b][3,4-b]thiadiazole derivatives may differ by various substituents in positions 3 and 6 such as alkyl-, aryl-, aryl(oxy)alkyl-, heteryl- etc. In this review, we presented the synthetic strategies and subsequent chemical transformations of the resulting triazolo[3,4-b]thiadiazoles, providing certain important class of functionalized compounds.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Maryan Lelyukh, Ivan Krukovskiy, Zoryana Komarenska, Nataliia Yelahina, Taras Chaban, Ihor Chaban

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
