Investigation of the Corrosion Inhibition of Carbon Steel in Acidic Solutions by One Schiff Base: Experimental and Theoretical Studies
DOI:
https://doi.org/10.17344/acsi.2025.9151Abstract
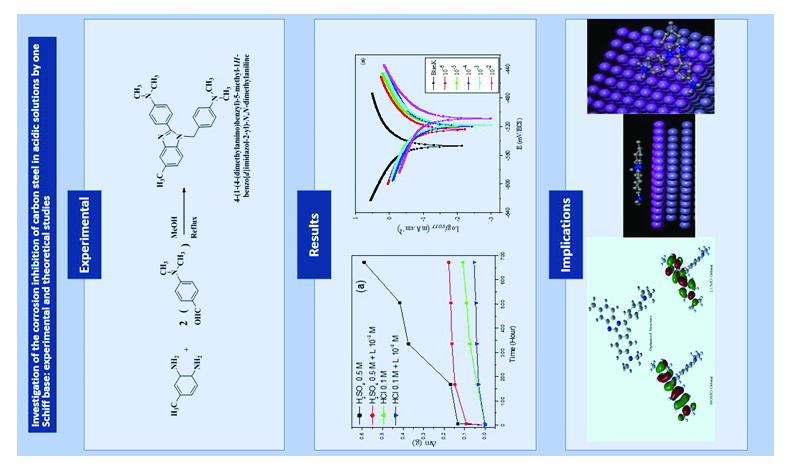
A new heterocyclic Schiff base, 4-(1-(4-(dimethylamino)benzyl)-5-methyl-1H-benzo[d]imidazol-2-yl)-N, N-dimethylaniline (L), has been synthesized and characterized. The molecular structure of compound L was confirmed by 1H NMR, FT-IR, UV-Vis and elemental analysis. The performance and inhibition mechanism of compound L against the corrosion of carbon steel in 1 M HCl and 0.5 M H2SO4 were studied by weight loss method, Tafel polarisation and electrochemical impedance spectroscopy (EIS). The compound behaves as a mixed-type inhibitor and obeys the Langmuir adsorption isotherm. The morphological appearance of compound L on the carbon steel surface by scanning electron microscopy (SEM) and atomic force microscopy (AFM) confirmed that compound L was adsorbed on the carbon steel to form a film. The thermodynamic parameters were calculated and discussed. Quantum chemical parameters were also determined. This study confirms the agreement between the experimental and theoretical results.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Djahida Haffar, Saida Mouzali, Linda Toukal, Youcef Bellal, Khadidja Boukerche, Abd El-Aziz S. Fouda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
