Syntheses, Crystal Structures and Antimicrobial Activity of NiII, ZnII and MnIII Complexes Derived from N,N’-Bis(4-bromosalicylidene)propane-1,2-diamine
DOI:
https://doi.org/10.17344/acsi.2024.9122Abstract
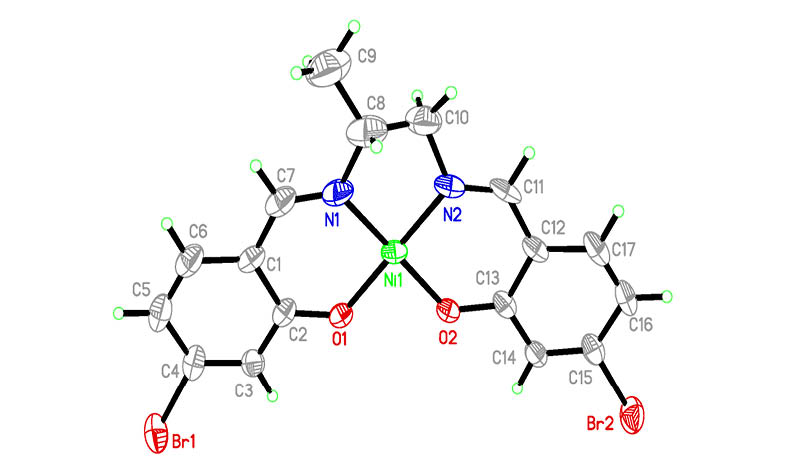
Four new nickel(II), zinc(II) and manganese(III) complexes, [NiL] (1), [Zn3L2(OAc)2] (2), [ZnL(CH3OH)] (3) and [MnClL(DMF)] (4), derived from the bis-Schiff base N,N’-bis(4-bromosalicylidene)propane-1,2-diamine (H2L) have been prepared and characterized by spectroscopy methods, as well as single crystal X-ray determination. The Ni atom in the mononuclear nickel complex 1 is in square planar coordination. The outer and inner Zn atoms in the trinuclear zinc complex 2 are in square planar and octahedral coordination, respectively. The Zn atom in the mononuclear zinc complex 3 is in square pyramidal coordination. The Mn atom in the mononuclear manganese complex 4 is in octahedral coordination. Antibacterial activity of the complexes has been assayed on the bacteria Staphylococcus aureus and Escherichia coli, and the yeast Candida parapsilosis.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Yu-Mei Hao

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
