Design, synthesis and anticonvulsant activity of 2 and 5-disubstituted 1,3-dioxoisoindoline
DOI:
https://doi.org/10.17344/acsi.2024.9007Abstract
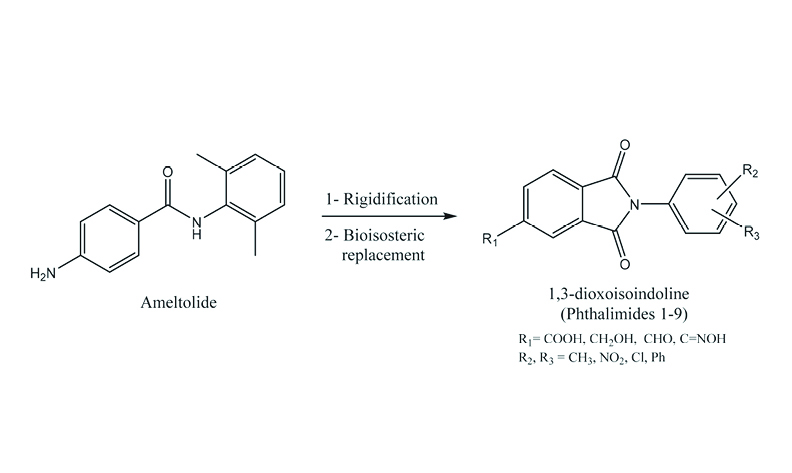
The isoindoline scaffold, a rigid analogue of ameltolide, exhibits notable antiepileptic properties. Here we describe the design, synthesis, and evaluation of nine new isoindoline derivatives prepared by condensation of trimellitic anhydride with various arylamines. Anticonvulsant activity of prepared compounds was assessed in maximal electroshock (MES; tonic seizure) and pentylenetetrazole (PTZ; clonic seizure) seizure models. All compounds significantly attenuated both tonic and clonic seizures; in MES they reduced seizure-induced mortality, while in PTZ they improved seizure frequency and latency. Compounds 3 and 4 showed the highest efficacy, surpassing phenytoin. Structure–activity analysis indicates that bulky ortho-substituents on the N-aryl group, combined with a meta-nitro substituent, enhance anticonvulsant potency.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Asghar Davood, Maryam Yadavar Nikravesh, Mahsa Hadipour Jahromy, Sepideh Taghizad

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
