Preparation, characterization and theoretical calculation of a cadmium complex of 3-hydroxy-2-methylquinolin-4-carboxylate and 1, 10-phenanthroline
DOI:
https://doi.org/10.17344/acsi.2024.9004Abstract
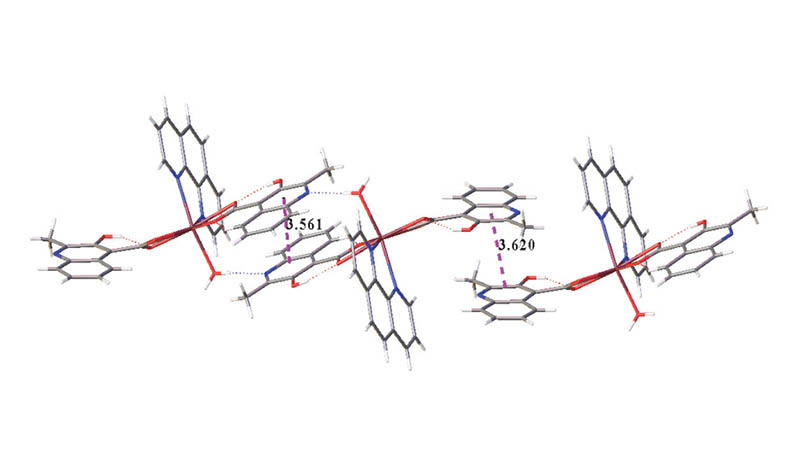
A novel organic-inorganic hybrid metal cadmium complex [Cd (MCA)2(Phen)(H2O)] (MCA = 3-hydroxyquinoline-4-carboxylic acid anion; Phen = 1,10-phenanthroline) was synthesized by solvothermal method and characterized by single crystal X-ray diffraction. The title complex exhibits a two-dimensional structure through hydrogen bonding and π···π packing interactions. Solid state photoluminescence spectra show that they exhibit red emission bands at 616 nm, and time-dependent density functional theory calculations show that they are ligand-to-ligand charge transfers. Solid diffuse reflection spectra show that the energy band gap of the complex is 2.32 eV.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Ting-Qun Qiu, Zhi-Tao Lu, Zi-Jian He, Zheng-Ping Xie, Jin Guo, Xiu-Guang Yi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
