Synthesis, Characterization and Thermal Decomposition Kinetics of a Novel Benzofuran Ketoxime Derived Polymer
DOI:
https://doi.org/10.17344/acsi.2014.900Keywords:
Synthesis, characterization, benzofuran, ketoxime, activation energy, thermogravimetric analysisAbstract
A novel benzofuran ketoxime derived polymer, poly(benzofuran-2-yl-methylketoxime-O-methacrylate) [poly(BMKMA], was firstly synthesized by free radical polymerization method. Its thermal degradation studies were performed by thermogravimetric analysis (TGA) in order to determine the actual reaction mechanisms of the decomposition process. The activation energy of the solid-state process was determined using Flynn-Wall-Ozawa method, which resulted to be 235.94 kJ/mol. The activation energies of different mechanism models were determined by Coats-Redfern, Madhusudanan and Van Krevelen kinetic methods. Compared with the Ozawa method, the actual reaction mechanism obeyed deceleration type, phase boundary controlled reaction (R1).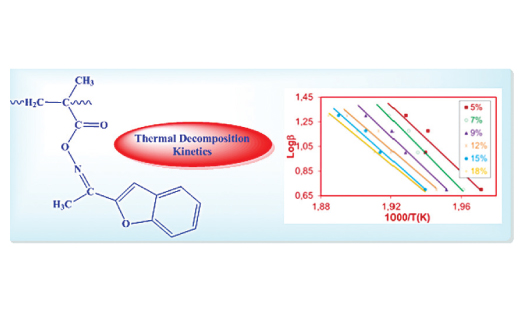
Downloads
Additional Files
Published
29.04.2015
Issue
Section
Physical chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
