The Effect of Fluorine Atom on the Synthesis and Composition of Gametocidal Ethyl Oxanilates
Keywords:
Chemical hybridizing agent, oxanilate, common wheat, heterosis, solvent-free synthesisAbstract
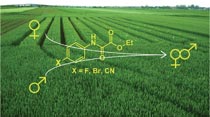
Three derivatives of ethyl oxanilate were synthesized in order to test their application as gametocides on the hermaphrodite plants like common wheat (Triticum aestivum L.). A substituent at para position (F, Br, CN) of aniline defined its reactivity towards diethyl oxalate 2. Classical reaction in toluene was not selective and amidation occurred also at the second carbonyl groups of 2. Alternative synthesis under solvent-free conditions with application of low pressure for removal of EtOH provided selectively with ethyl oxanilate 3a and 3b. 4-Cyanoaniline did not react selectively and the corresponding ethyl oxanilate 3c was prepared from mono acid chloride of oxalic acid. Fluoro derivative 3a was found to be the only one that gives stable aqueous suspension for its application as chemical hybridizing agent for common wheat, while bromo- 3b and cyano- 3c analogues were not soluble enough and suspension was stable for less than 2 hours. Fluoro derivative had shown the best induction of male sterility, while in comparison with standard chemical hybridizing agent they were substantially less toxic for plant.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
