Syntheses, Crystal Structures and Characterization of Two New Lanthanide Mercury Halide Compounds
DOI:
https://doi.org/10.17344/acsi.2024.8937Abstract
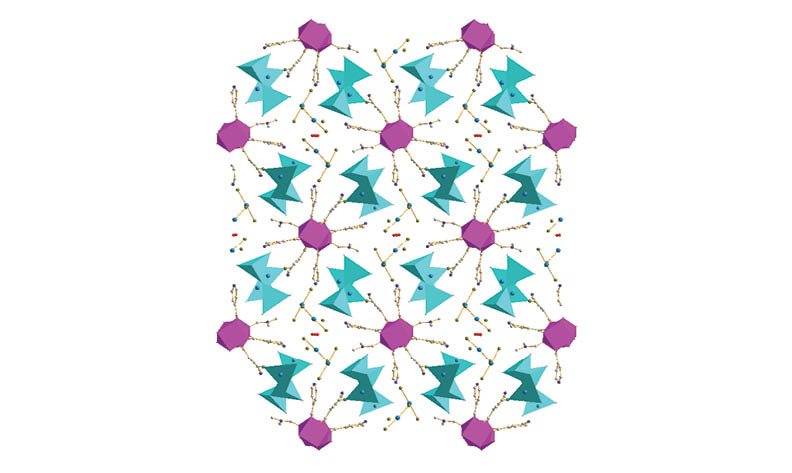
Two new lanthanide mercury halide compounds with isonicotinic acid as a ligand, namely, [Gd(HIA)2(IA)(H2O)2(HgCl2)]n(nHgCl4)·3nH2O (1) (HIA = isonicotinic acid) and {[Nd(HIA)3(DMF)(H2O)]n}[(Hg4Br11)n](2HgBr2)(nBr)·nH3O·0.5nH2O (2) (DMF = N,N′-Dimethylformamide), were synthesized by means of solvothermal reactions and characterized by single-crystal X-ray diffraction. Compound 1 is characterized by a two-dimensional (2-D) layer-like structure, while compound 2 features a one-dimensional (1-D) chain-like structure. The lanthanide ions in both compounds are eight coordination and show a square antiprism geometry. The mercury ions exhibit various coordination motifs. Compound 1 shows a ultraviolet upconversion photoluminescence emission, while compound 2 displays red photoluminescence. The photoluminescence emissions come from the characteristic emissions of the 4f electron intrashell transitions of the 6P7/2 → 8S7/2 of the Gd3+ ions in compound 1 and the 4F9/2 → 4I9/2, 4F7/2 + 4S3/2→ 4I9/2, 4F5/2 + 2H9/2→ 4I9/2, 4F3/2 → 4I9/2 of the Nd3+ ions in compound 2. Compound 2 has CIE (Commission Internationale de I'Éclairage) chromaticity coordinates (0.7142, 0.2857) and its CCT (correlated color temperature) is 138224 K. Solid-state UV/Vis diffuse reflectance spectra discovered that their semiconductor band gaps are 3.12 eV and 3.23 eV, respectively.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Xi-Yu Shao, Hao-Dong Liu, Long-Hua Zeng, Yu-Yue Xu, Wen-Tong Chen, Cheng Liu, Sheng-Ping Dai, Chang-Wang Pan

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
