Inhibition Effect of Benzimidazole Derivatives on the Corrosion of Mild Steel in Acidic Medium: Experimental and Theoretical Studies
DOI:
https://doi.org/10.17344/acsi.2024.8922Abstract
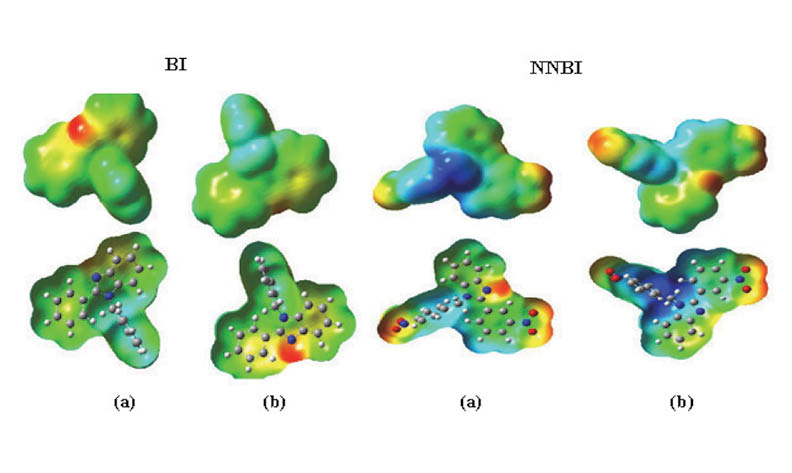
The effect of heterocyclic compounds, derived from benzimidazole (BnZ), namely1-Benzyl-2-phenyl 1H-benzimidazole (BI) and1-(4-Nitrobenzyl)-2-(4-nitrophenyl)-1H-benzimidazole(NNBI),on the carbon corrosion steel in 1M HCl medium was assessed by electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP). The effects of the concentration and the temperature were studied. The determined electrochemical parameters showed that the two inhibitors are of mixed type. The inhibitory effect of NNBI was lesser compared to that of BI. The most electron-withdrawing substituent offers the lowest efficiency. The mechanism of action of these inhibitors has been defined by the thermodynamic study. Calculated,, and values confirmed that BI and NNBI adsorb through a chemical and physical process. The adsorption process was found to be spontaneous and followed the Langmuir adsorption isotherm. The quantum chemical parameters calculated by density functional theory (DFT) and molecular dynamics simulation (MDS) corroborate with both the experimental data and those of the literature.
Keywords: Corrosion; steel; organic inhibitors; benzimidazole; DFT; MDS.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Sonia Benabid, Linda Toukal

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
