Acetylacetone: Is the β-Diketone Tautomer Less Stable Than the Enol or Is the Enol Tautomer More Stable Than the Diketone?
DOI:
https://doi.org/10.17344/acsi.2024.8870Abstract
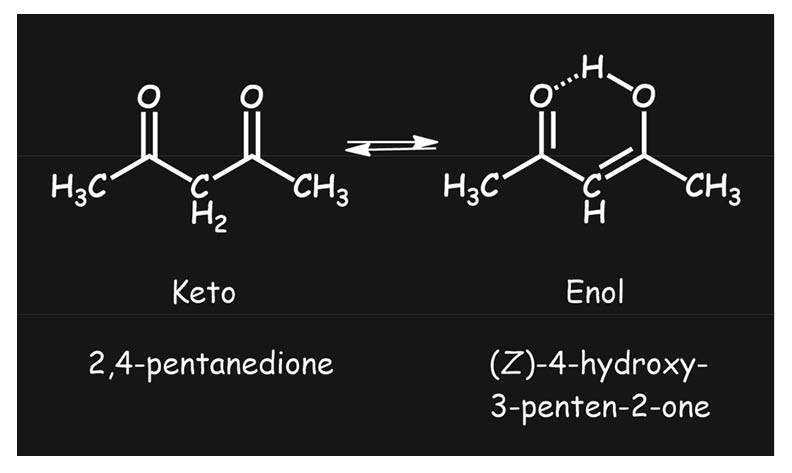
Tautomerism is a fundamental concept that studies the transfer of a proton between two constitutional isomers, i.e. keto and enol tautomers, where the enol tautomers are usually less stable than the corresponding carbonyl compound. However, this is not the case with acetylacetone. This article discusses two rapidly inter-converting tautomeric forms of acetylacetone, the archetypal (and now textbook) example of tautomerism in organic chemistry from a thermochemical perspective.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Maja Ponikvar-Svet, Kathleen F. Edwards, Joel F. Liebman

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
