Extraction System for the Spectrophotometric Determination of Tungsten(VI) with 4-Nitrocatechol and Benzalkonium Chloride
DOI:
https://doi.org/10.17344/acsi.2024.8835Abstract
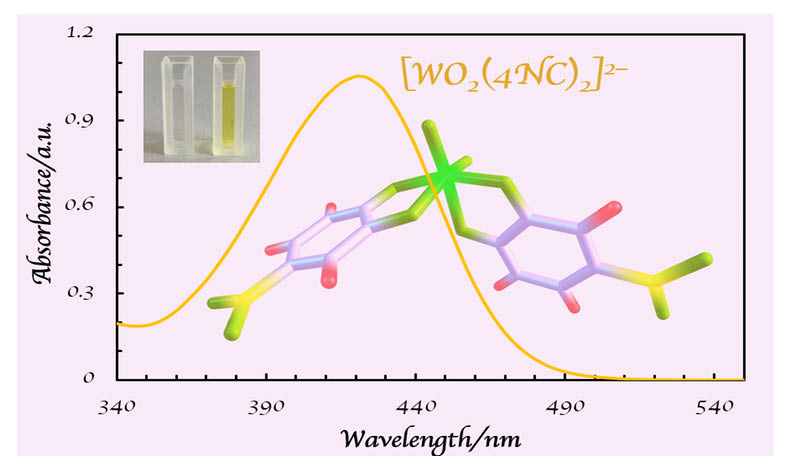
A novel chromogenic system for the liquid-liquid extraction and determination of trace amounts of tungsten(VI) was investigated. The system comprises 4-nitrocatechol (4NC) as a chromogenic reagent, sulfuric acid as a complexing medium, and benzalkonium chloride (BAC) as a source of bulky cations (BA+), which readily form chloroform-extractable ion-association complexes. The impact of foreign ions and reagents was studied, and the optimal conditions for the sensitive, selective, and inexpensive determination of tungsten(VI) were identified. The limit of detection, linear working range, and molar absorptivity at lmax (422 nm) were determined to be 31 ng cm−3, 0.1–4.4 µg cm−3, and 5.49 × 104 dm3 mol−1 cm−1, respectively. The composition of the extracted complex was 1:2:2 (W:4NC:BA). Two potential structures of its anionic component, [WO2(4NC)2]2–, were discussed based on optimizations at the B3LYP/CEP-4G theoretical level and comparison between theoretical and experimental spectra.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Vidka Vassileva Divarova, Kirila Trifonova Stojnova, Ivelina Dobromirova Radkovska, Antoaneta Dimitrova Saravanska, Galya Kostadinova Toncheva, Vassil Borisov Delchev, Kiril Blazhev Gavazov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
