The effect of tyrosine residues on α-synuclein fibrillation
DOI:
https://doi.org/10.17344/acsi.2014.882Keywords:
α-synuclein, fibrillation, Parkinson’s disease, site-directed mutagenesis, ThT fluorescence, tyrosineAbstract
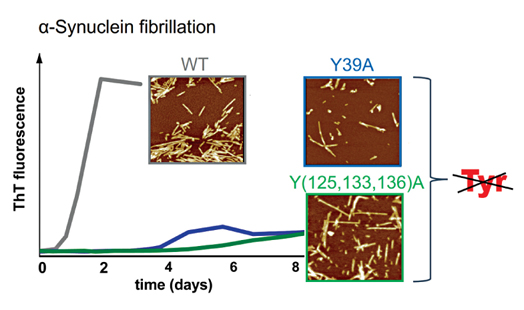
Aggregation of the intrinsically disordered protein α-synuclein into ordered amyloid fibrils is implicated in the pathogenesis of Parkinson’s disease. To unravel the role of Tyr residues in α-synuclein fibrillation, we prepared recombinant N-terminal (Y39A) and C-terminal (Y(125,133,136)A) mutants of α-synuclein and examined their fibrillation propensities by thioflavin T and 1-anilinonaphthalene-8-sulfonate (ANS) fluorescent probes, SDS-PAGE and atomic force microscopy. We demonstrate that in contrast to wild-type α-synuclein, both mutants show large, but comparable delays in the fibrillation process and exhibit enhanced hydrophobicity during fibril-like assembly. Both Tyr mutants form fibril-like structures after prolonged incubation periods, which are morphologically distinct from those of the wild-type protein. Our results suggest that the N-terminal and C-terminal Tyr residues of α-synuclein are important primarily for the initiation of the fibrillation process.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
