The Effect of Fluoride Ions on the Corrosion Behaviour of Ti Metal, and Ti6–Al–7Nb and Ti–6Al–4V Alloys in Artificial Saliva
Keywords:
Dental alloys, artificial saliva, corrosion, X-ray photoelectron spectroscopy, ICP-MSAbstract
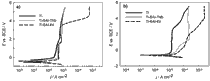
Metallic materials used for manufacture of dental implants have to exhibit high corrosion resistance in order to prevent metal release from a dental implant. Oral cavity is aggressive towards metals as it represents a multivariate environment with wide range of conditions including broad range of temperatures, pH, presence of bacteria and effect of abrasion. An increasing use of various Ti-based materials for dental implants and orthodontic brackets poses the question of their corrosion resistance in the presence of fluoride ions which are present in toothpaste and mouth rinse. Corrosion behaviour of Ti metal, Ti–6Al–7Nb and Ti–6Al–4V alloys and constituent metals investigated in artificial saliva is significantly affected by the presence of fluoride ions (added as NaF), as proven by electrochemical methods. Immersion test was performed for 32 days. During that time the metal dissolution was measured by inductively coupled plasma mass spectrometry. At the end of the test the composition, thickness and morphology of the surface layers formed were investigated by X-ray photoelectron spectroscopy and scanning electron microscopy.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
