Synthesis of Bifunctional Amine-Squaramide Organocatalysts Derived from 3-((Dimethylamino)methylene)camphor
DOI:
https://doi.org/10.17344/acsi.2024.8757Abstract
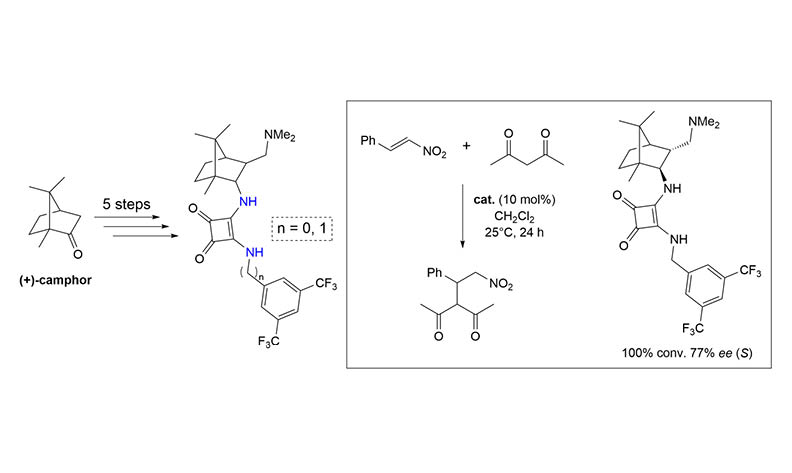
Four bifunctional, noncovalent amine-squaramide organocatalysts were prepared from camphor in five steps. The stereochemistry of the prepared catalysts was thoroughly analyzed using various spectroscopic techniques. Their organocatalytic activity was investigated in the Michael addition of acetylacetone to trans-β-nitrostyrene. The addition product was formed in complete conversion and with an enantioselectivity of up to 77% ee. In the reactions catalyzed by the 2-exo-3-endo catalysts, the major (S)-enantiomer was formed, whereas in the presence of 2-endo-3-endo catalysts, the (R)-enantiomer was formed as the major product.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Uroš Grošelj, Luka Ciber, Helena Brodnik, Franc Požgan, Jurij Svete, Bogdan Štefane

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
