1H-Indole-2,3-dione 3-thiosemicarbazones carrying a 4-sulfamoylphenyl moiety with selective antiviral activity against reovirus-1
Isatin thiosemicarbazones with selective action against Reovirus-1
DOI:
https://doi.org/10.17344/acsi.2023.8589Abstract
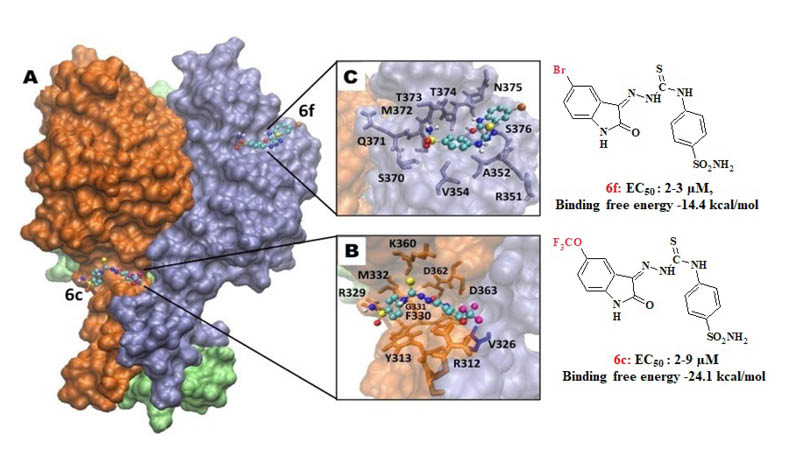
1H-indole-2,3-dione 3-[4-(4-sulfamoylphenyl)thiosemicarbazones] (6a-j) were evaluated against Para-influenza-3, Reovirus-1, Sindbis, Coxsackie B4 and Punto Toro viruses. New 1-methyl-1H-indole-2,3-dione 3-[4-(4-sulfamoylphenyl)thiosemicarbazones] (7a-c) were synthesized to evaluate the contribution of methyl substitution at position 1- of the indole ring to antiviral activity. The test results showed that compounds 5-trifluoromethoxy- substituted 6c (EC50: 2-9 µM) and 5-bromo- substituted 6f (EC50: 2-3 µM) have non-toxic selective antiviral activity while not all standards are active against Reovirus-1. Molecular docking studies of 6c and 6f were carried out to determine the possible binding positions with Reovirus-1. Trifluoromethoxy and bromine substitutions at position 5- of the indole ring provided selective antiviral activity, while methyl substitution at position 1- of the indole ring significantly decreased the activity and increased toxicity against Reovirus-1.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Füsun Göktaş, Gizem Nur Duran, Mehmet Özbil, Özge Soylu-Eter, Nilgün Karalı

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
