Syntheses, Crystal Structures and Antimicrobial Activity of Zinc(II) Complexes Derived from 5-Bromo-2-(((2-piperazin-1-yl)ethyl)imino)methyl)phenol
DOI:
https://doi.org/10.17344/acsi.2023.8588Abstract
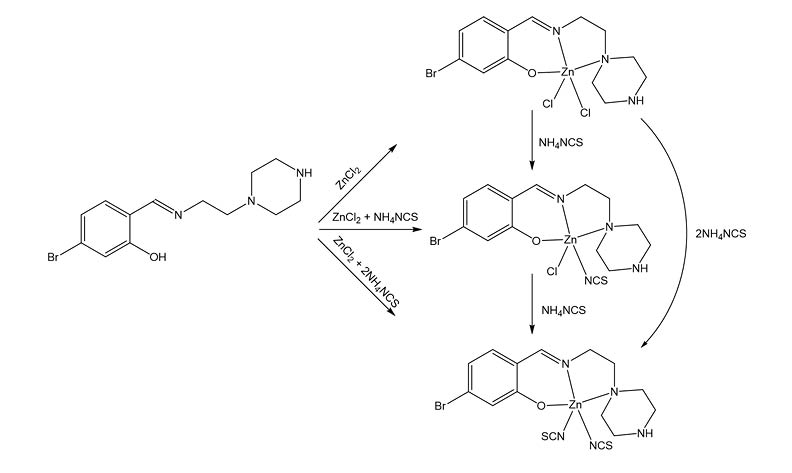
Three new zinc(II) complexes, [ZnCl2L]·CH3OH (1), [ZnClL(NCS)]·2CH3OH·0.5H2O (2), and [ZnL(NCS)2]·CH3OH·H2O (3), where L is the zwitterionic form of 5-bromo-2-(((2-piperazin-1-yl)ethyl)imino)methyl)phenol, NCS is thiocyanate anion, were facile prepared by reaction of different molar ratio of L, zinc chloride and ammonium thiocyanate in methanol. The complexes were characterized by IR and UV-Vis spectroscopy. Detailed structures of the three complexes were confirmed by single crystal X-ray determination. The Zn atoms in the complexes are in tetrahedral coordination. The Schiff base ligand coordinates to Zn atom through phenolate oxygen and imino nitrogen. The remaining two sites are occupied by two Cl for 1, one Cl and one NCS for 2, and two NCS for 3. The compounds show significant antimicrobial activities.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Xiao-Yang Qiu, Yin-Bing Chen, Meng-Yuan Xu, Fei-Yu Qi, Xin He, Chen Wu, Shu-Juan Liu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
