Synthesis, antibacterial and antibiofilm activity of new 1,2,3,5-tetrazine derivatives from coupling reactions of diazonium salt of 2-amino-6-nitrobenzothiazole with diverse substituted 2-aminobenzothiazole derivatives
DOI:
https://doi.org/10.17344/acsi.2023.8550Abstract
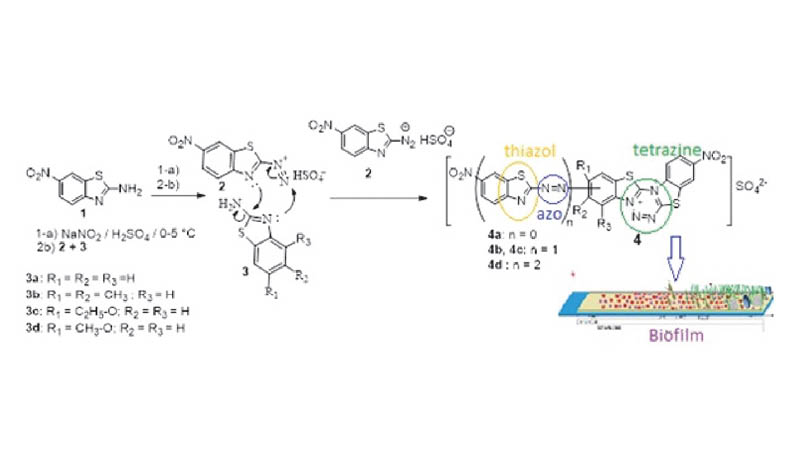
The coupling reaction of diazonium ion of 2-amino-6-nitrobenzothiazole at 0-5 °C with distinctly substituted 2-aminobenzothiazole derivatives produced new 1,2,3,5-tetrazine derivatives. It was found that diazotized 2-amino-6-nitrobenzo[d]thiazol reacts with the ring nitrogen atom of varyingly substituted 2-aminobenzothiazole derivatives to yield tetrazine nucleus. The benzene ring of benzothiazole bearing electron donor group and annelated to the tetrazine was further substituted in situ by other 6-nitrobenzo[d]thiazol-2-yl) diazinyl to yield the final product. The structure of the prepared compounds was elucidated using their physical, elemental, and spectroscopic data. The synthesized compounds were tested for their antimicrobial and antibiofilm activities against Staphylococcus aureus and Escherichia coli bacteria. Two of the synthesis tetrazine derivatives exhibited interesting antibiofilm potential.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Joseph Tsemeugne, Yetiny Atuh Bah, Ulrich Joel Tsopmene, Armelle Tontsa Tsamo, Jérôme Ndefo Ndefonganga, Pierre Mkounga, Emmanuel Fondjo Sopbué, Jean Paul Dzoyem, Augustin Ephrem Nkengfack

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
