Surface Passivation of Natural Graphite Electrode for Lithium Ion Battery by Chlorine Gas
Keywords:
Surface modification, chlorine gas, chlorination, natural graphite electrode, lithium ion batteryAbstract
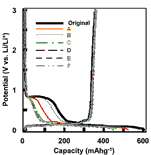
Surface lattice defects would act as active sites for electrochemical reduction of propylene carbonate (PC) as a solvent for lithium ion battery. Effect of surface chlorination of natural graphite powder has been investigated to improve charge/ discharge characteristics of natural graphite electrode in PC-containing electrolyte solution. Chlorination of natural graphite increases not only surface chlorine but also surface oxygen, both of which would contribute to the decrease in surface lattice defects. It has been found that surface-chlorinated natural graphite samples with surface chlorine concentrations of 0.5–2.3 at% effectively suppress the electrochemical decomposition of PC, highly reducing irreversible capacities, i.e. increasing first coulombic efficiencies by 20–30% in 1 molL–1 LiClO4–EC/DEC/PC (1:1:1 vol.). In 1 molL–1 LiPF6–EC/EMC/PC (1:1:1 vol.), the effect of surface chlorination is observed at a higher current density. This would be attributed to decrease in surface lattice defects of natural graphite powder by the formation of covalent C–Cl and C=O bonds.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
