Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculations of the Novel Binuclear Copper(I) Complex Containing 2-Benzimidazolethiole and Triphenylphosphine Ligands
DOI:
https://doi.org/10.17344/acsi.2023.8416Abstract
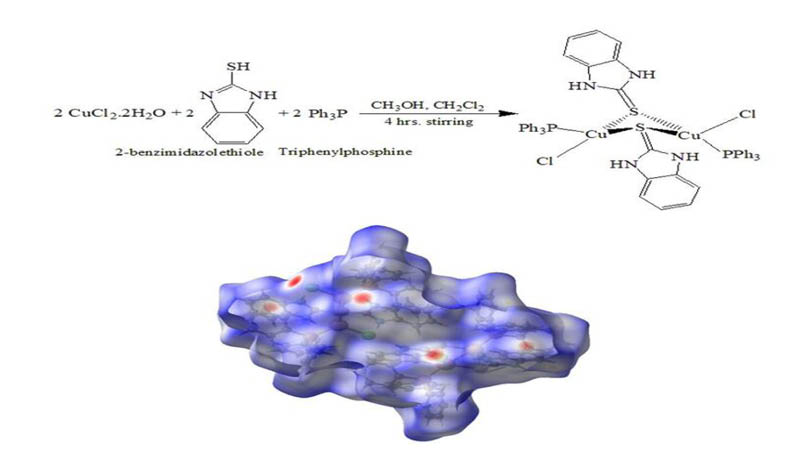
The reaction of 2-benzimidazolethiole (2-BIT) with copper dichloride in the presence of two equivalents of triphenylphosphine led to a binuclear complex of the type [Cu(µ-S-2-BIT)2(Ph3P)2Cl2]: dichloridobis(μ-1,3-dihydro-2H-benzimidazole-2-thione)bis(triphenylphosphi ne)-di-copper. The Cu(I) compound has been fully identified by elemental analysis, molar conductivity, FT-IR, UV/Vis, and single-crystal X-ray diffraction (XRD). The XRD study reveals that the complex has distorted tetrahedral geometry around the Cu(I)center, which contains two bridge sulfur atoms. The Hirshfeld surface mapped over dnorm, shape index, and curvature revealed important H...H, H... C/C...H, and H... Cl/Cl...H intermolecular interactions as the main contributors to crystal packing. The natural bond orbital (NBO) was applied to understand the strength of nucleophilic and electrophilic attack between ligands and Cu(I) ions. Furthermore, density functional theory (DFT) was employed to demonstrate the molecular reactivity and stability of the ligands and copper complex.
Downloads
Published
Issue
Section
License
Copyright (c) 1970 Karwan Omer Ali

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
